The Quantum Fridge
It all comes down to quantum physics: scientists at TU Wien (Vienna) have analysed why some gases can be cooled down to extremely low temperatures.
Copyright: TU Wien
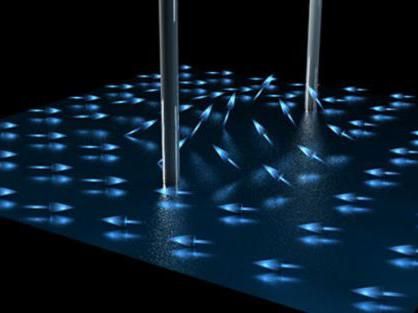
When cold milk is poured into a hot cup of tea, a temperature equilibrium is reached very quickly. The milk droplets and the tea particles interact, and after a few moments they all have the same average energy. This process is called thermalization. It plays a crucial role in cooling down gases to ultra-low temperatures. But surprisingly, even gases for which this effect is suppressed can be cooled. Scientists at TU Wien (Vienna) took a closer look at this phenomenon and found a special quantum-mechanical kind of cooling at work.
Kicking Out Hot Particles
“The particles that make up a liquid or a gas have different energies”, says Professor Jörg Schmiedmayer (TU Wien). The distribution of these energies depends on the temperature. The hotter the gas, the higher the number of particles with high energies. Therefore, a simple trick can be used to cool down cold gases: with the help of electromagnetic fields, the particles with the highest energy are removed from the gas. The remaining ones interact, redistribute the energy, and the gas relaxes again to a typical energy distribution—but at a slightly lower temperature than before.
“It is a bit like blowing on your tea in order to cool it”, says Bernhard Rauer, who led the experiments in Schmiedmayer’s research group. “The particles with the highest energy manage to leave the liquid and are blown away. The remaining tea quickly reaches an equilibrium state at a slightly lower temperature.”
However, there are cases, in which reaching such a thermal equilibrium is not possible. A Newton’s cradle for example is a device that has several spheres hanging from strings, arranged in a straight line. When one of the spheres is set in motion and hits the others, the last sphere in line is kicked away, while the others do not move. “In this case, the spheres can only exchange energies between each other. There will never be a thermal distribution of many different energies”, says Bernhard Rauer.
Rauer studied a similar system: a one dimensional gas of atoms, kept in a straight line by an electromagnetic trap. The atoms can just exchange their energies, just like the spheres in Newton’s cradle. Therefore, one would expect the cooling mechanism of removing the most energetic particles to fail in this case. When the fastest particles are gone, no other particles in the gas will ever have the same speed again. According to this simple model, an energy that is missing will be gone forever.
Astonishingly, this is not true for the one-dimensional gas. It can be cooled down by continuously removing particles—to much lower energies than one would expect, according, to the simplified picture of fast and slow particles.
It’s a Wave!
The reason for this effect is that the particles can only be understood quantum mechanically. “We should not think of individual particles colliding like the spheres in Newton’s cradle. Instead, we have to consider collective excitations, which are distributed across many particles – like a water wave, which is carried by many water molecules at the same time”, says Jörg Schmiedmayer. These quantum waves store the energy of the system, and the more particles that are removed from the gas, the less intense these waves become. This is a quantum mechanical cooling mechanism which should be impossible according to simple classical laws of nature.
“For us it is crucial that the gas behaves more and more quantum mechanically at low temperatures”, says Jörg Schmiedmayer. “That is exciting, because that is precisely what we are interested in: often quantum physics is studied in simple systems, consisting only of a few particles, such as an atom with a few electrons. We have a system which undeniably exhibits quantum behaviour, and it is made of thousands of atoms.”
Most read news
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.