Visualizing atoms of perovskite crystals
Organic-inorganic perovskite materials are key components of the new generation of solar cells. Understanding properties of these materials is important for improving lifetime and quality of solar cells. Researchers from the Energy Materials and Surface Sciences (EMSS) Unit, led by Prof. Yabing Qi, at the Okinawa Institute of Science and Technology Graduate University (OIST) in collaboration with Prof. Youyong Li's group from Soochow University (China) and Prof. Nam-Gyu Park's group from Sungkyunkwan University (Korea) report the first atomic resolution study of organic-inorganic perovskite.
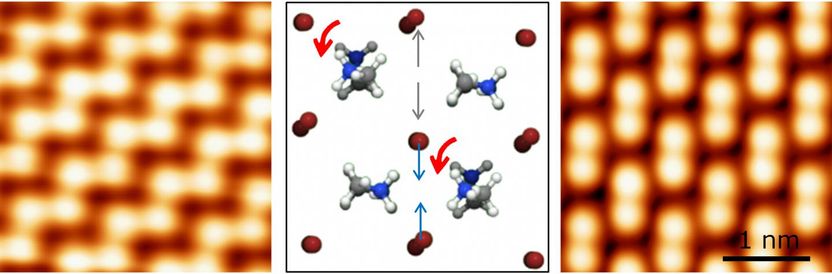
Methylammonium molecules (represented by a ball-and-stick model in the centre) in methylammonium lead bromide (CH3NH3PbBr3) can rotate. They favour specific orientations that lead to two types of surface structures with distinctly different properties (left and right images).
OIST
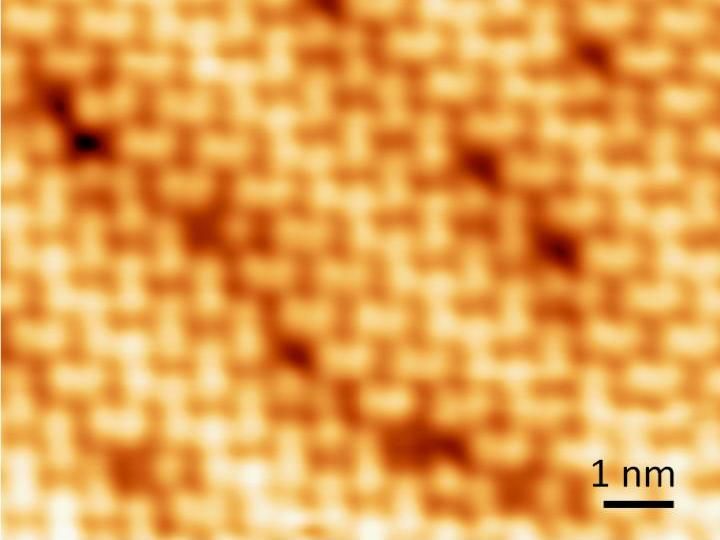
Atomic size defects on the surface of a perovskite crystal.
OIST
Perovskites are a class of materials with the general chemical formula ABX3. A and B are positive ions bound by negative ions X. Organic-inorganic perovskites used in solar cells are usually methylammonium lead halides (CH3NH3PbX3, where X is bromine, iodine, or chlorine). The OIST scientists used a single crystal of methylammonium lead bromide (CH3NH3PbBr3) to create topographic images of its surface with a scanning tunneling microscope.
This microscope uses a conducting tip that moves across the surface in a manner very similar to a finger moving across a Braille sign. While the bumps in Braille signs are a few millimetres apart, the microscope detects bumps that are more than million times smaller -- atoms and molecules. This is achieved by the quantum tunneling effect -- the ability of an electron to pass through a barrier. The probability of an electron passing between the material surface and the tip depends on the distance between the two. The resulting atomic-resolution topographic images reveal positions and orientations of atoms and molecules, and also provide a detailed look at structural defects in the surface.
"At room temperature atoms and molecules are quite mobile, so we decided to freeze the crystal to almost absolute zero (-269ºC) to get a good picture of its atomic structure," says Dr Robin Ohmann, a member of the EMSS Unit and the first author of the paper. The crystal was cut and studied in a vacuum to avoid contamination of the surface. Dr Ohmann's colleagues from Soochow University calculated atomic structures using principles of quantum physics and then compared them with scanning tunneling microscopy data.
The researchers discovered that methylammonium molecules can rotate and that they favour specific orientations that lead to two types of surface structures with distinctly different properties. Apart from rotation, these molecules affect positions of neighbouring bromine ions, further altering the atomic structure. Since the structure dictates the electronic properties of the material, the geometric positions of atoms are essential for understanding of solar cells.
Scanning tunneling microscope images also reveal local imperfections caused by dislocations of molecules and ions and, probably, missing atoms. These imperfections may affect device performance, for example, by changing electrical properties such as conductivity.
The structure of perovskite materials is temperature-sensitive and the observed structure of the frozen crystal might not be fully identical to the structure at room temperature. However, the comprehensive description of perovskite crystals at the atomic level paves the way to better understanding of their behaviour under real-life conditions. The current findings shed light on molecule-ion interplay on the surface of an organic-inorganic crystal and should help to improve designs of future solar cells. The next goal of the researchers is to examine interactions between perovskites and other molecules, for example water molecules that are known to interfere with the performance of solar cells.




























































