Surface physics: How water learns to dance
Perovskites are materials used in batteries, fuel cells, and electronic components, and occur in nature as minerals. Despite their important role in technology, little is known about the reactivity of their surfaces. Professor Ulrike Diebold's team at TU Wien (Vienna) has answered a long-standing question using scanning tunnelling microscopes and computer simulations: How do water molecules behave when they attach to a perovskite surface? Normally only the outermost atoms at the surface influence this behaviour, but on perovskites the deeper layers are important, too.
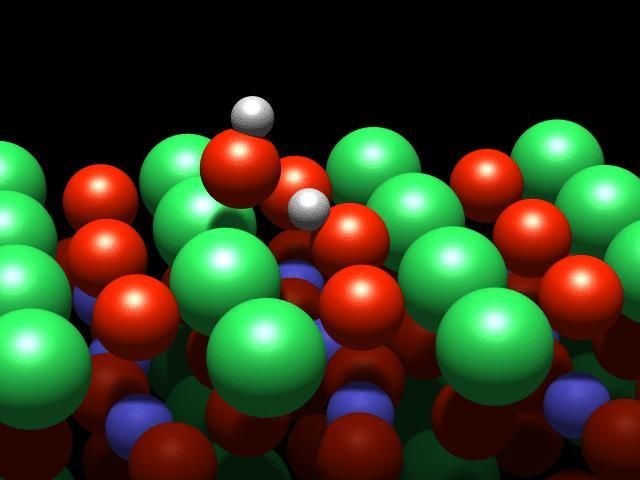
This is a visualization of the dance of the atoms on a crystal surface.
TU Wien
Perovskite dissociates water molecules
"We studied strontium ruthenate - a typical perovskite material," says Ulrike Diebold. It has a crystalline structure containing oxygen, strontium and ruthenium. When the crystal is broken apart, the outermost layer consists of only strontium and oxygen atoms; the ruthenium is located underneath, surrounded by oxygen atoms.
A water molecule that lands on this surface splits into two parts: A hydrogen atom is stripped off the molecule and attaches to an oxygen atom on the crystal's surface. This process is known as dissociation. However, although they are physically separated, the pieces continue to interact through a weak "hydrogen bond".
It is this interaction that causes a strange effect: The OH group cannot move freely, and circles the hydrogen atom like a dancer spinning on a pole. Although this is the first observation of such behaviour, it was not entirely unexpected: "This effect was predicted a few years ago based on theoretical calculations, and we have finally confirmed it with our experiments" said Diebold.
Dancing requires space
When more water is put on to the surface, the stage becomes too crowded and spinning stops. "The OH group can only move freely in a circle if none of the neighbouring spaces are occupied," explains Florian Mittendorfer, who performed the calculations together with PhD student Wernfried Mayr-Schmölzer. At first, when two water molecules are in neighbouring sites, the spinning OH groups collide and get stuck together, forming pairs. Then, as the amount of water is increased, the pairs stick together and form long chains. Eventually, water molecular cannot find the pair of sites it needs to split up, and attaches instead as a complete molecule.
The new methods that have been developed and applied by the TU Wien research team have made significant advances in surface research. Whereas researchers were previously reliant on indirect measurements, they can now - with the necessary expertise - directly map and observe the behaviour of individual atoms on the surface. This opens up new possibilities for modern materials research, for example for developing and improving catalysts.
Original publication
Most read news
Original publication
Daniel Halwidl, Bernhard Stöger, Wernfried Mayr-Schmölzer, Jiri Pavelec, David Fobes, Jin Peng, Zhiqiang Mao, Gareth S. Parkinson, Michael Schmid, Florian Mittendorfer, Josef Redinger & Ulrike Diebold; "Adsorption of water at the SrO surface of ruthenates"; Nature Materials; 2015
Topics
Organizations
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Tetraspanin
Ultra-compact phase modulators based on graphene plasmons
RasMol
Metabolically engineered E. coli producing phenol
Category:Protein_targeting


























































