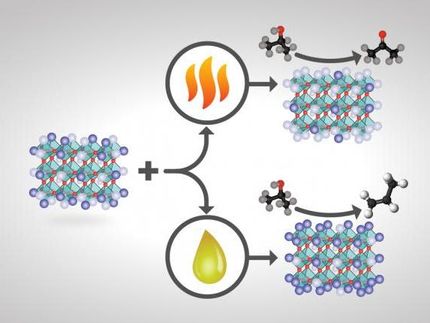
Cooperative catalysts offer unique route to alkenes
Advertisement
Chemists at Princeton have developed a new chemical method to introduce valuable alkenes into simple hydrocarbon molecules, a transformation known as dehydrogenation, which is found in important processes such as the biosynthesis of essential fatty acids in the body and the commercial production of detergents.
Leading approaches in the natural and synthetic classes of reactions possess attractive features that are unavailable to the other. Existing synthetic methods produce hydrogen gas as a useful byproduct but require very high temperatures, while natural methods run at mild temperatures but produce a full equivalent of an unusable byproduct.
"What if we could have a method that offers the benefits of both approaches? Can we be greedy?" asked Julian West, a graduate student in the Sorensen lab. Guided by this goal, the researchers devised a novel two-component catalyst system that performs the dehydrogenation reaction at room temperature, making hydrogen gas and a molecule containing an alkene, or carbon-carbon double bond.
"If you're a chemist, you have a keen sense of the importance of the alkene," Erik Sorensen, the Arthur Allan Patchett Professor in Organic Chemistry at Princeton and corresponding author. Alkenes are highly versatile and can readily serve as starting materials in many reactions. "It's a portal to a whole range of compound types," he said.
The alkene was generated using a pair of catalysts to separately remove two hydrogen atoms from neighboring carbons atoms. To break the first, stronger carbon-hydrogen bond, the researchers chose a so-called hydrogen atom transfer catalyst known as decatungstate, which is activated by ultraviolet light. "We used light energy to get the reaction going," Sorensen said.
Once the first bond is broken, the adjacent hydrogen atom was much easier to remove using a second catalyst, a compound called cobaloxime. "It's like a one-two punch," West said, "once we had the substrate on the ropes, we didn't need a very strong catalyst to take the other hydrogen atom."
Another advantage of the method is that both catalysts are made of cheap, earth abundant metals, instead of the precious metals, such as iridium and rhodium, used in current state-of-the-art methods. However, the dual catalyst system has yet to reach the same level of efficiency as existing methods, which the researchers suspect is due to the alkene product binding to the decatungstate catalyst, inhibiting its participation in the reaction.
"The method is noted more for its conceptual uniqueness than its efficiency," Sorensen said.
It's the dual catalyst system design that could really "pay dividends," West said. The researchers plan to apply of their strategy to other chemical transformations as well. "We're all really excited because this reaction opens the door to catalysis for our group," he said.
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.