Boosting solid state chemical reactions
Adding olefin enables efficient solvent-free cross-coupling reactions, leading to environmentally friendly syntheses of a wide range of organic materials.
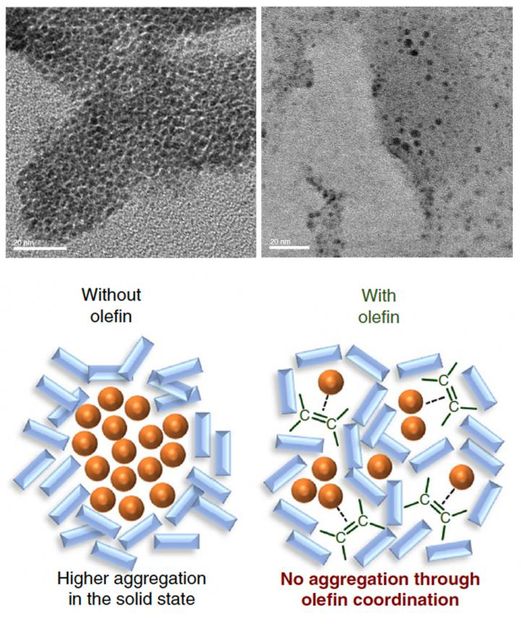
Top: Electron microscopic images of palladium nanoparticles in the reaction mixtures. After 99 minutes of the reaction, the palladium catalyst was found aggregated and deactivated itself (left) while the addition of olefin kept the catalyst dispersed (right). Bottom: Schematic illustration showing the function of olefin as a dispersant
Kubota K. et al., Nature Communications, Jan. 10, 2019
A cross coupling reaction is typically performed in an organic solvent and leads to the production of a large amount of solvent waste, which is often harmful to the environment. A new strategy developed by Hokkaido University researchers in Japan opens the door for more environmentally friendly solvent-free solid-state cross coupling processes using mechanochemistry. It also has many potential applications, including the development of organic materials found in solar cells and light-emitting diodes.
Cross-coupling reactions proceed efficiently in the presence of a metal catalyst to form a wide range of organic molecules with novel properties. In particular, the Nobel-prize-winning palladium-catalysed cross-coupling reactions have long been used in the synthesis of natural products, in medicinal chemistry, and in polymer and materials science.
To reduce environmental waste, researchers have been looking at methods to enable efficient organic syntheses that use less or no solvent. In this context, "solid-state organic transformations" have received considerable research attention, but improving the efficiency of cross-coupling reactions in solid media remains a challenge.
In a study, Hokkaido University organic chemists Koji Kubota, Hajime Ito and their colleagues developed a new strategy for solid-state palladium-catalysed cross-coupling reactions using mechanochemistry that enables efficient solvent-free synthesis of organic materials.
Two solid organic materials were placed inside a ball milling jar that contains a stainless steel grinding ball. A palladium-based catalyst was also added. The jar undergoes a shaking process that causes the ball to grind the solid compounds, initiating a cross-coupling reaction.
They found that the palladium-based catalyst tended to aggregate during the reaction, which may lead to catalyst deactivation. But, when olefin such as 1,5-cyclooctadiene was added to the mixture, it acted as a dispersant for the palladium-based catalyst, facilitating a more efficient solid-state cross-coupling reaction. When olefin was added, the conversion rate of the reaction went up from less than 30% to 99%.
"Our protocol should be particularly useful for reducing the amount of organic solvent used in industry that is harmful to the environment. It will also make the production process less costly," said Hajime Ito. "The new method could be applied to, for example, the production of triarylamines that can be found in a wide range of organic materials including solar cells and light-emitting diodes."
Original publication
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
George_Frederick_Kunz
Squeezing low-cost electricity from sustainable biomaterial
Arnaldus_de_Villa_Nova
Aristides_Brezina

European Consortium Launches DELYCIOUS Research Project to Optimize Operation of Renewable Hydrogen Production Units