Electronics get a power boost with the addition of a simple material
The tiny transistor is the heart of the electronics revolution, and Penn State materials scientists have just discovered a way to give this workhorse a big boost, using a new technique to incorporate vanadium oxide into the electronic devices.
"It's tough to replace current transistor technology because semiconductors do such a fantastic job," said Roman Engel-Herbert, assistant professor of materials science and engineering. "But there are some materials, like vanadium oxide, that you can add to existing devices to make them perform even better."
The researchers knew that vanadium dioxide, which is just a specific combination of the elements vanadium and oxygen, had an unusual property called the metal-to-insulator transition. In the metal state, electrons move freely, while in the insulator state, electrons cannot flow. This on/off transition, inherent to vanadium dioxide, is also the basis of computer logic and memory.
The researchers thought that if they could add vanadium oxide close to a device's transistor it could boost the transistor's performance. Also, by adding it to the memory cell, it could improve the stability and energy efficiency to read, write and maintain the information state. The major challenge they faced was that vanadium dioxide of sufficiently high quality had never been grown in a thin film form on the scale required to be of use to industry - the wafer scale.
Although vanadium dioxide, the targeted compound, looks simple, it is very difficult to synthesize. In order to create a sharp metal-to-insulator transition, the ratio of vanadium to oxygen needs to be precisely controlled. When the ratio is exactly right, the material will show more than four orders-of-magnitude change in resistance, enough for a sufficiently strong on/off response.
The Penn State team reports in Nature Communications that they are the first to achieve growth of thin films of vanadium dioxide on 3-inch sapphire wafers with a perfect 1 to 2 ratio of vanadium to oxygen across the entire wafer. The material can be used to make hybrid field effect transistors, called hyper-FETs, which could lead to more energy efficient transistors.
The implementation of vanadium dioxide can also benefit existing memory technologies, a quest that Penn State researchers are actively pursuing.
"The metal-to-insulator property of vanadium dioxide can ideally enhance state-of-the-art non-volatile memories by using the material as an augmentation device, which, interestingly, can also serve as a selector in some memory architecture," said Sumeet Gupta, Monkowski Assistant Professor of Electrical Engineering and group leader of the Integrated Circuits and Devices Lab, Penn State.
A selector insures that reading or writing information on a memory chip is done within a single memory cell, without bleeding over into neighboring cells. The selector works by changing the resistivity of the cell, which vanadium dioxide does extremely well. In addition, the change in resistivity of vanadium oxide can be used to significantly increase the robustness of the read operation.
"To determine the right ratio of vanadium to oxygen, we applied an unconventional approach in which we simultaneously deposit vanadium oxide with varying vanadium-to-oxygen ratios across the sapphire wafer," said Hai-Tian Zhang, Ph.D. student in Engel-Herbert's group. "Using this 'library' of vanadium-to-oxygen ratios, we can perform flux calculations to determine the optimal combination that would give an ideal 1 to 2 vanadium to oxygen ratio in the film. This new method will allow a rapid identification of the optimal growth condition for industrial applications, avoiding a long and tedious series of trial-and-error experiments."
The vanadium dioxide thin-film material grown with this method was used to make super-high-frequency switches, in collaboration with the Datta group at Penn State and Notre Dame, a technology important in communications. These switches show cut-off frequencies an order of magnitude higher than conventional devices.
Most read news
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Prandtl-Meyer_function
Carbenicillin
K&N_Engineering,_Inc.
Super-sensitive detection of asbestos in soil samples
Liquid_crystal_on_silicon
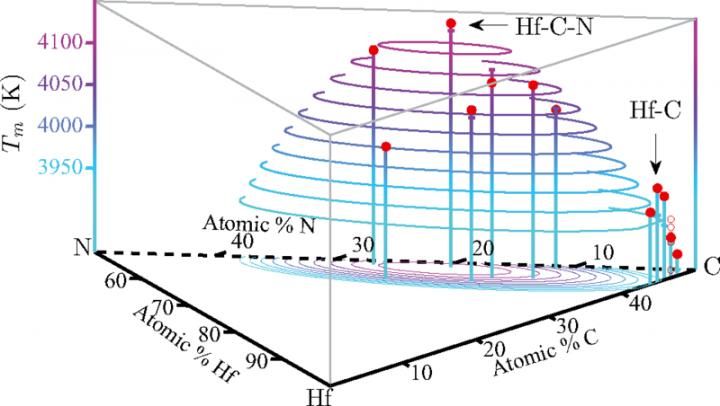
Researchers predict material with record-setting melting point
Gadoversetamide
Terazosin
Surfaces get smooth or bumpy on demand - MIT research produces soft material with controllable surface textures that can be varied by squeezing