Chalmers researchers extend the lifetime of atoms using a mirror
Researchers at Chalmers University of Technology have succeeded in an experiment where they get an artificial atom to survive ten times longer than normal by positioning the atom in front of a mirror.
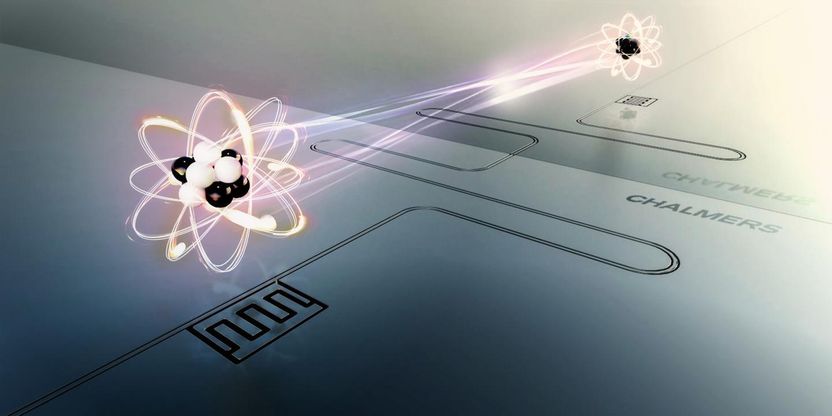
The lifetime of an atom can be extended up to ten times by placing it in front of a short circuit that acts as a mirror. The artificial atom consists of a superconducting circuit on a silicon chip. The interaction between the atom and its mirror image modifies the vacuum fluctuations seen by the atom and thus its lifetime.
Moa Carlsson and Lisa Kinnerud, Krantz NanoArt
If one adds energy to an atom - one says that the atom is excited - it normally takes some time before the atom loses energy and returns to its original state. This time is called the lifetime of the atom. Researchers at Chalmers University of Technology have placed an artificial atom at a specific distance in front of a short circuit that acts as a mirror. By changing the distance to the mirror, they can get the atom to live longer, up to ten times as long as if the mirror had not been there.
The artificial atom is actually a superconducting electrical circuit that the researchers make behave as an atom. Just like a natural atom, you can charge it with energy; excite the atom; which it then emits in the form of light particles. In this case, the light has a much lower frequency than ordinary light and in reality is microwaves.
"We have demonstrated how we can control the lifetime of an atom in a very simple way," says Per Delsing, Professor of Physics and leader of the research team. "We can vary the lifetime of the atom by changing the distance between the atom and the mirror. If we place the atom at a certain distance from the mirror the atom's lifetime is extended by such a length that we are not even able to observe the atom. Consequently, we can hide the atom in front of a mirror," he continues.
The experiment is a collaboration between experimental and theoretical physicists at Chalmers, the latter have developed the theory for how the atom's lifetime varies depending on the distance to the mirror.
"The reason why the atom "dies", that is it returns to its original ground state, is that it sees the very small variations in the electromagnetic field which must exist due to quantum theory, known as vacuum fluctuations," says Göran Johansson, Professor of Theoretical and Applied Quantum Physics and leader of the theory group.
When the atom is placed in front of the mirror it interacts with its mirror image, which changes the amount of vacuum fluctuations to which the atom is exposed. The system that the Chalmers researchers succeeded in building is particularly well suited for measuring the vacuum fluctuations, which otherwise is a very difficult thing to measure.
Original publication
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Mycophenolic_acid
Fuchsine