Flipping molecular attachments amps up activity of CO2 catalyst
Advertisement
New research by chemists at the U.S. Department of Energy's Brookhaven National Laboratory and their collaborators offers clues that could help scientists design more effective catalysts for transforming CO2 to useful products. The study reveals how a simple rearrangement of molecular attachments on an iridium hydride catalyst can greatly improve its ability to coax notoriously stable CO2 molecules to react.
Brookhaven National Laboratory
The research, which combined laboratory experiments with theoretical analysis, shows that, in the dark, only one of the two molecular arrangements can effectively transform CO2 to formate, a precursor of methanol. In the presence of light, however, both species form a common intermediate that can transform CO2 to CO, a useful raw material for making fuels and industrial chemicals.
"There is strong interest in finding ways to reuse CO2 to create a carbon-neutral society," said Brookhaven chemist Etsuko Fujita, who led the experimental portion of this work. "Reactions to produce products such as methanol or hydrocarbons from CO2 would be very useful. But if you think about the energy input and output of these reactions, it's really very difficult," she said.
Finding more efficient catalysts is the key to lowering the energy required to jump-start these reactions. Because various researchers had suggested that the iridium hydride catalyst might be an improvement over other well-known catalysts for producing CO from CO2, Fujita's group undertook this research to investigate its mechanism of action.
"If you understand how a catalyst works, you can often devise ways to modify its function to make it work even better," said Zahid Ertem, whose theoretical analyses provided the framework for understanding the experimental results.
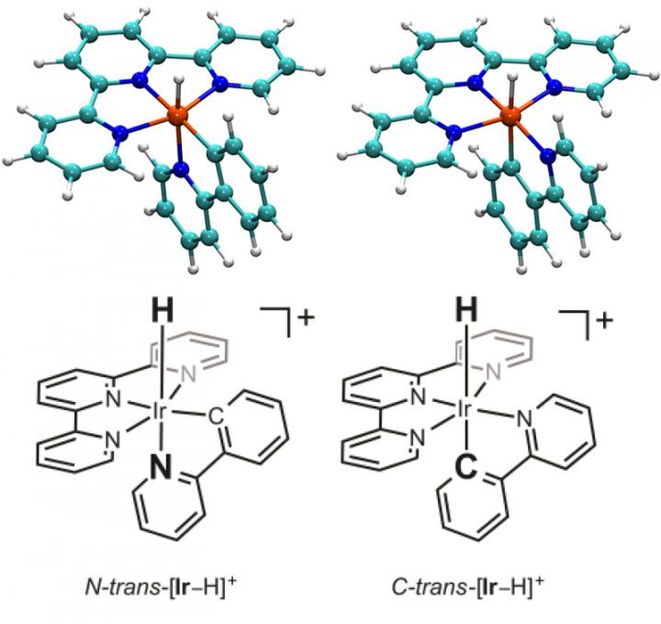
The only structural difference between the two isomers is a simple flip in positioning of two connected rings of atoms relative to the rest of the molecule-one linked to the central iridium atom by a negatively charged carbon atom, and the other linked by a neutral nitrogen atom. But that simple flip in the positions of these two rings has a dramatic effect on the respective molecules' properties.
For example, the molecule was much more likely to give up what's called a hydride ion-a negatively charged ion consisting of a proton and two electrons-when the carbon-linked ring was in a position opposite the hydride compared with when the nitrogen-linked ring was in that position.
"That's important because the chemical conversion of CO2 to other products such as hydrocarbons can be facilitated when electrons are transferred to CO2 two at a time as they are in hydride ions," Ertem said. "So a catalyst with a higher tendency to release these hydride ions-a higher hydricity-is potentially a much better catalyst.
"While the generation of formate is the first step in a possible sequence of hydride transfer reactions, we envision going further than formate-for example, to methanol," he said.
Another aim of the study was to explore the role of iridium hydride as a proposed key intermediate in the conversion of CO2 to CO. But as it turns out, the intermediate is a form of the molecule that lacks the hydride but has the carbon-linked ring in the position opposite where the hydride would attach.
"In fact, no matter which isomer we started with, the theoretical calculations show that this species with the carbon positioned opposite the vacant hydride position forms as an intermediate, which then catalyzes the conversion of CO2 to CO," Ertem said.
"Because that intermediate is so reactive," Fujita added, "it is extremely hard to isolate experimentally-which is one reason the theoretical analysis was so important to this study. The theoretical analysis corroborated all the measurements we could make and predicted the existence of this one key intermediate," she said.
Original publication
Dr. Komal Garg, Dr. Yasuo Matsubara, Dr. Mehmed Z. Ertem, Dr. Anna Lewandowska-Andralojc, Dr. Shunsuke Sato, Dr. David J. Szalda, Dr. James T. Muckerman and Dr. Etsuko Fujita; "Striking Differences in Properties of Geometric Isomers of [Ir(tpy)(ppy)H]+: Experimental and Computational Studies of their Hydricities, Interaction with CO2, and Photochemistry"; Angew. Chem. Int. Ed.; 2015