Tiny magnets mimic steam, water and ice
Advertisement
Researchers at the Paul Scherrer Institute (PSI) created a synthetic material out of 1 billion tiny magnets. Astonishingly, it now appears that the magnetic properties of this so-called metamaterial change with the temperature, so that it can take on different states; just like water has a gaseous, liquid and a solid state. This material made of nanomagnets might well be refined for electronic applications of the future – such as for more efficient information transfer.
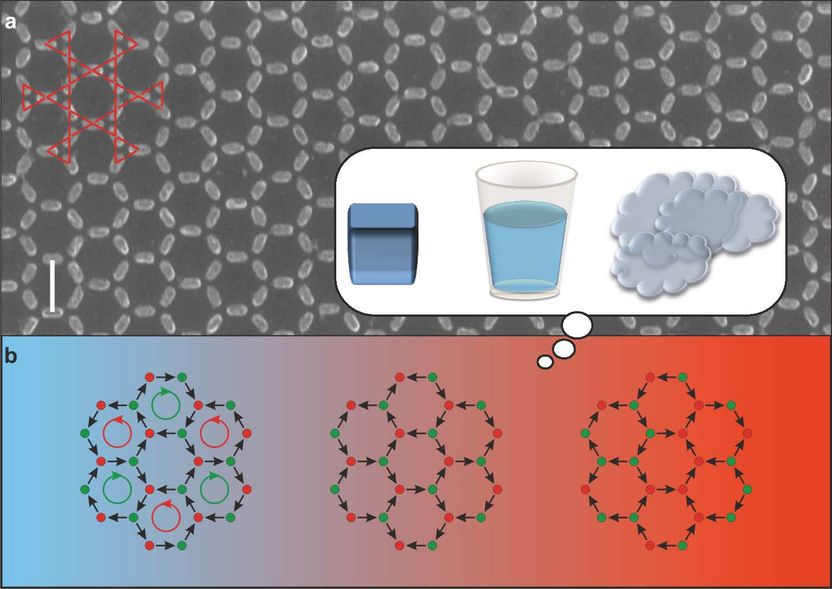
PSI researchers have created a synthetic magnetic metamaterial. Depending on the temperature it behaves similarly to ice, water and steam.
Paul Scherrer Institut/Luca Anghinolfi
A synthetic material – created from 1 billion nanomagnets – assumes different aggregate states depending on the temperature: the so-called metamaterial exhibits phase transitions, much like those between steam, water and ice. This effect was observed by a team of researchers headed by Laura Heyderman from PSI. “We were surprised and excited,” explains Heyderman. “Only complex systems are able to display phase transitions.” And as complex systems can provide new kinds of information transfer, the result of the new study also reveals that the PSI researchers’ metamaterial would be a potential candidate here.
The major advantage of the synthetic metamaterial is that it can be customised virtually freely. While the individual atoms in a natural material cannot be rearranged with pinpoint precision on such a grand scale, the researchers say that this is possible with the nanomagnets.
Honeycomb of nanomagnets
The magnets are only 63 nanometres long and shaped roughly like grains of rice. The researchers used a highly advanced technique to place 1 billion of these tiny grains on a flat substrate to form a large-scale honeycomb pattern. The nanomagnets covered a total area of five by five millimetres.
Thanks to a special measuring technique, the scientists initially studied the collective magnetic behaviour of their metamaterial at room temperature. Here there was no order in the magnetic orientation: the magnetic north and south poles pointed randomly in one direction or another.
When the researchers cooled the metamaterial gradually and constantly, however, they reached a point where a higher order appeared: the tiny magnets now noticed each other more than before. As the temperature fell further, there was another change towards an even higher order, in which the magnetic arrangement appeared almost frozen. The long-range order of water molecules increases in a similar way at the moment when water freezes into ice. “We were fascinated by the fact that our synthetic material displayed this everyday phenomenon of a phase transition,” says Heyderman.
Metamaterial can be customised
In the next step, the researchers might influence these magnetic phase transitions by altering the size, shape and arrangement of the nanomagnets. This enables the creation of new states of matter, which could also give rise to applications: “The beauty of it all: tailored phase transitions could enable metamaterials to be adapted specifically for different needs in future,” explains Heyderman.
Besides its potential use in information transfer, the metamaterial might also prove useful in data storage or for sensors that measure magnetic fields. Very generally it could be used in spintronics, so in a promising future development in electronics for novel computer technology.
The measurements the researchers used to reveal the magnetic orientation of the nanomagnets, and therefore the properties of the metamaterial, can only be conducted exclusively at PSI. The equipment at the SμS, which is unique worldwide, supplies beams from exotic elementary particles called muons, which can be used to study nanomagnetic properties. The project took place in collaboration with a research group headed by Stephen Lee from the University of St Andrews, Scotland.