Soaking up carbon dioxide and turning it into valuable products
Berkeley Lab researchers double down on a good thing by incorporating catalysts into crystalline sponges
A molecular system for the capture and storage of carbon dioxide has been modified so that it now also holds great promise as a catalyst for converting captured carbon dioxide into valuable chemical products. Researchers with the U.S. Department of Energy's Lawrence Berkeley National Laboratory have incorporated molecules of carbon dioxide reduction catalysts into the sponge-like crystals of covalent organic frameworks (COFs). This creates a molecular system that not only absorbs carbon dioxide, but also selectively reduces it to carbon monoxide, which serves as a primary building block for a wide range of chemical products including fuels, pharmaceuticals and plastics.
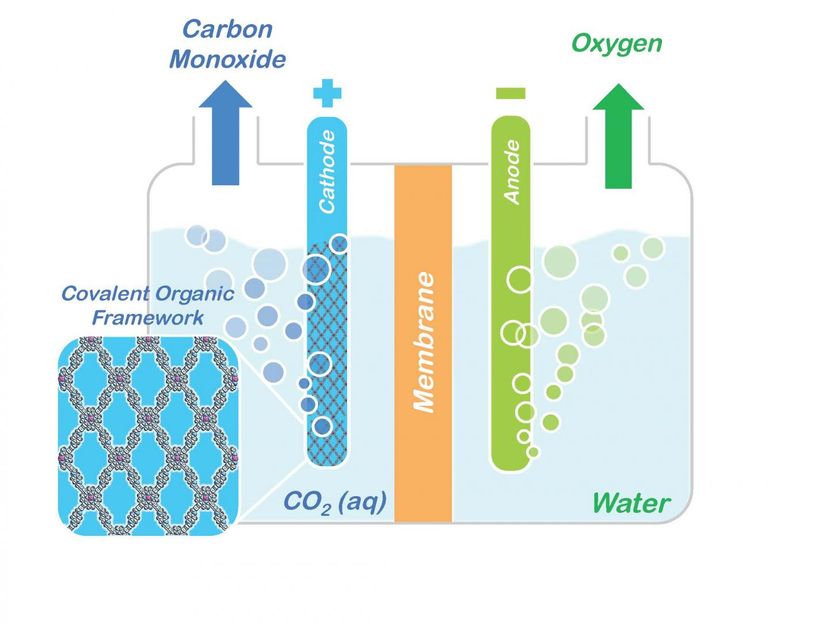
Conceptual model shows how porphyrin COFs embedded in a cathode could be used to split carbon dioxide (CO2) into carbon monoxide (CO) and oxygen for making renewable fuels and other valuable chemical products.
Courtesy of Omar Yaghi, Berkeley Lab/UC Berkeley
"There have been many attempts to develop homogeneous or heterogeneous catalysts for carbon dioxide, but the beauty of using COFs is that we can mix-and-match the best of both worlds, meaning we have molecular control by choice of catalysts plus the robust crystalline nature of the COF," says Christopher Chang, a chemist with Berkeley Lab's Chemical Sciences Division, and a co-leader of this study. "To date, such porous materials have mainly been used for carbon capture and separation, but in showing they can also be used for carbon dioxide catalysis, our results open up a huge range of potential applications in catalysis and energy."
Chang and Omar Yaghi both hold appointments with the University of California (UC) Berkeley. Chang is also a Howard Hughes Medical Institute (HHMI) investigator. Yaghi is co-director of the Kavli Energy NanoScience Institute (Kavli-ENSI) at UC Berkeley.
With the reduction of atmospheric carbon dioxide emissions in mind, Yaghi and his research group at the University of Michigan designed and developed the first COFs as a means of separating carbon dioxide from flue gases.Now, through another technique developed by Yaghi, called "reticular chemistry," which enables molecular systems to be "stitched" into netlike structures that are held together by strong chemical bonds, the Berkeley Lab researchers were able to embed the molecular backbone of COFs with a porphyrin catalyst, a ring-shaped organic molecule with a cobalt atom at its core. Porphyrins are electrical conductors that are especially proficient at transporting electrons to carbon dioxide.
"A key feature of COFs is the ability to modify chemically active sites at will with molecular-level control by tuning the building blocks constituting a COF's framework," Yaghi says. "This affords a significant advantage over other solid-state catalysts where tuning the catalytic properties with that level of rational design remains a major challenge. Because the porphyrin COFs are stable in water, they can operate in aqueous electrolyte with high selectivity over competing water reduction reactions, an essential requirement for working with flue gas emissions."
Original publication
Most read news
Original publication
Song Lin, Christian S. Diercks, Yue-Biao Zhang, Nikolay Kornienko, Eva M. Nichols, Yingbo Zhao, Aubrey R. Paris, Dohyung Kim, Peidong Yang, Omar M. Yaghi, Christopher J. Chang; "Covalent organic frameworks comprising cobalt porphyrins for catalytic CO2 reduction in water"; Science Express; 2015
Organizations
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Metabolically engineered E. coli producing phenol
Category:Protein_targeting


























































