Study calculates the speed of ice formation
Advertisement
Researchers at Princeton University have for the first time directly calculated the rate at which water crystallizes into ice in a realistic computer model of water molecules. The simulations provide insight into the mechanism by which water transitions from a liquid to a crystalline solid.
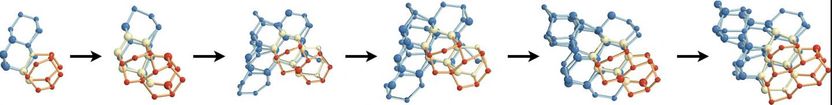
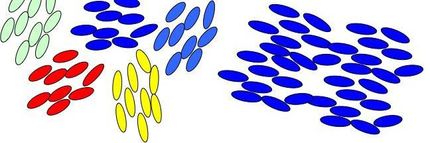
Using a computer model to explore how water molecules connect and nucleate into ice crystals, the researchers found that two types of ice compete for dominance during nucleation: cubic ice (blue) which is less stable, and hexagonal ice (red), which is stable and forms the majority of ice on Earth. Nucleation occurs when water molecules come together to form blobs (pictured above), which grow over time (left to right). Eventually hexagonal ice wins out (not shown). The researchers found that adding new cubic features onto an existing crystalline blob gives rise to nuclei that are more spherical, and hence more stable. In contrast, adding hexagonal features tends to give rise to chains of hexagonal cages that make the nucleus less spherical, and hence less stable.
Amir Haji-Akbari, Princeton University
Understanding ice formation adds to our knowledge of how cold temperatures affect both living and non-living systems, including how living cells respond to cold and how ice forms in clouds at high altitudes. A more precise knowledge of the initial steps of freezing could eventually help improve weather forecasts and climate models, as well as inform the development of better materials for seeding clouds to increase rainfall.
The researchers looked at the process by which, as the temperature drops, water molecules begin to cling to each other to form a blob of solid ice within the surrounding liquid. These blobs tend to disappear quickly after their formation. Occasionally, a large enough blob, known as a critical nucleus, emerges and is stable enough to grow rather than to melt. The process of forming such a critical nucleus is known as nucleation.
To study nucleation, the researchers used a computerized model of water that mimics the two atoms of hydrogen and one atom of oxygen found in real water. Through the computer simulations, the researchers calculated the average amount of time it takes for the first critical nucleus to form at a temperature of about 230 Kelvin or minus 43° Celsius, which is representative of conditions in high-altitude clouds.
They found that, for a cubic meter of pure water, the amount of time it takes for a critical nucleus to form is about one-millionth of a second. The study was conducted by Amir Haji-Akbari, a postdoctoral research associate, and Pablo Debenedetti, a professor of chemical and biological engineering.
"The main significance of this work is to show that it is possible to calculate the nucleation rate for relatively accurate models of water," said Haji-Akbari.
In addition to calculating the nucleation rate, the researchers explored the origin of the two different crystalline shapes that ice can take at ambient pressure. The ice that we encounter in daily life is known as hexagonal ice. A second form, cubic ice, is less stable and can be found in high-altitude clouds.
Computer models come in handy for studies of nucleation because conducting experiments at the precise temperatures and atmospheric conditions when water molecules nucleate is very difficult, said Debenedetti, who is Princeton's Class of 1950 Professor in Engineering and Applied Science.
Haji-Akbari found a way to complete the calculation, whereas previous attempts failed to do so. The technique for modeling ice formation involves looking at computer-simulated blobs of ice, known as crystallites, as they form. Normally the technique involves looking at the crystallites after every step in the simulation, but Haji-Akbari modified the procedure such that longer intervals of time could be examined, enabling the algorithm to converge to a solution and obtain a sequence of crystallites that eventually led to the formation of a critical nucleus.
Debenedetti noted that the rate of ice formation obtained in their calculations is much lower than what had been found by experiment. However, the computer calculations are extremely sensitive, meaning that small changes in certain parameters of the water model have very large effects on the calculated rate. The researchers were able to trace the discrepancy, which is 10 orders of magnitude, to aspects of the water model rather than to their method. As the modeling of water molecules improves, the researchers may be able to refine their calculations of the rate.