The risk potential of hormonally active substances is to be comprehensively characterised
BfR proposes further EU criteria to identify endocrine disruptors
On the occasion of the EU Conference "Endocrine disruptors: criteria for identification and related impacts" held in Brussels on 1 June 2015, the Federal Institute for Risk Assessment (BfR) presented an extended proposal for the identification of endocrine disruptors. This identification is to be based on a complex decision matrix. "Health considerations must be given top priority in the regulation of hormonally active substances", says BfR President Professor Dr. Dr. Andreas Hensel. "For this reason, the BfR recommends comprehensive characterisation of the risk potential of substances in the identification of endocrine disruptors." According to EU law, endocrine disruptors, i.e. substances that due to their hormonal effects can adversely impact human and animal health, must be banned as active substances in plant protection products in the future.
Scientists distinguish between endocrine substances and endocrine disruptors, since hormonally active substances do not necessarily have an adverse effect on health. According to the definition of the World Health Organisation (WHO), an endocrine disruptor is an exogenous substance or mixture that alters function(s) of the endocrine system and consequently causes adverse health effects in an intact organism, or its progeny, or (sub)populations.
The decision matrix proposed by the BfR for health assessment suggests that assessing the strength (“potency”) of endocrine disruptors in terms of their hormonal impact alone is not sufficient, even though this approach is currently still recommended in one of the options of the EU roadmap. Rather, the BfR proposal aims to significantly enhance this option and to take into account additional criteria. These include the severity and reversibility of adverse health effects, i.e. the possibility of complete recovery from such effects; the specificity of the hormonal properties of a substance against the background of further possible properties; as well as consistency, i.e. the scientific traceability of the studies. These decision criteria, potency, severity, reversibility, specificity and consistency, then serve as a template for classifying substances into one of the three categories “Hormonally active substance”, “Suspected endocrine disruptor” and “Endocrine disruptor”. On the basis of these categories, it will also be possible to make regulatory decisions as proposed by the EU roadmap, for example by banning endocrine disruptors in pesticides and biocides.
At the end of 2014, the European Commission commissioned the DG Health and Food Safety to conclusively define criteria for the identification of endocrine disruptors in order to use those criteria in European pesticide and biocide law. Due to an increasing concern worldwide about the potentially harmful effects of hormonally active substances, it is planned that within the EU, active ingredients contained in pesticides and biocides requiring registration will be tested more rigorously for damaging effects on the hormonal system. In the discussion, the proposal of the BfR received wide support from conference participants and within the EU Commission. The international conference organised by DG Health and Food Safety was attended by representatives of the EU member states as well as other countries, members of the European Parliament and representatives of business, unions, non-government organisations and the media.
Most read news
Organizations
Related link
Other news from the department politics & laws

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
It's official! Element 113 was discovered at RIKEN
Cryptographic potential in nanomaterial discovered - The ultimate defense against hackers may be just a few atoms thick

Analytica 2020: The leading guide to the smart laboratory - The focus on digital transformation is being further expanded
Category:Flavin_enzymes
Joint effort for new detector technologies - Helmholtz Association funds DESY-China cooperation for ATLAS upgrade

Self-organizing molecules: Nanorings with two sides
Metallic five-membered ring pushes the boundaries of aromaticity - Researchers succeed in synthesizing a molecule that should not actually exist
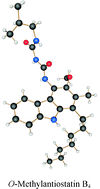
Building up antioxidants
Glucose_transporter
Monoamine_oxidase