Probing iron chemistry in the deep mantle
carbonates are a group of minerals that contain the carbonate ion (CO32-) and a metal, such as iron or magnesium. Carbonates are important constituents of marine sediments and are heavily involved in the planet's deep carbon cycle, primarily due to oceanic crust sinking into the mantle, a process called subduction. During subduction, carbonates interact with other minerals, which alter their chemical compositions. The concentrations of the metals gained by carbonate ions during these interactions are of interest to those who study deep earth chemistry cycles.
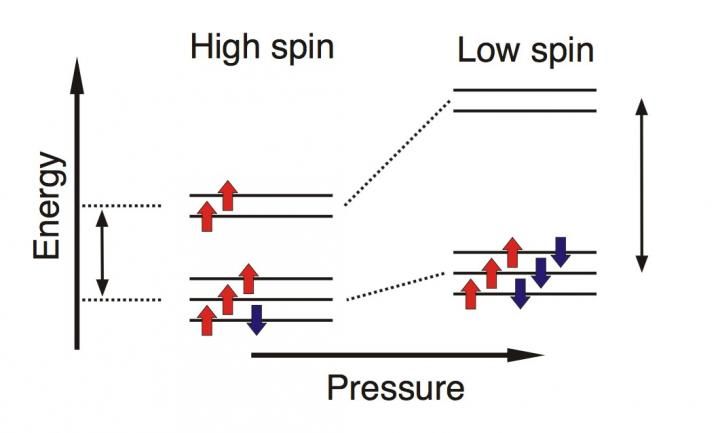
Rearrangement of the electrons in iron upon pressure-induced spin transition in carbonates.
Sergey Lobanov
Carbonates were known to exist in the upper mantle due to their role in the deep carbon cycle. But it was thought that they could not withstand the more-extreme conditions of the lower mantle. Laboratory experiments and the discovery of tiny bits of carbonate impurities in lower mantle diamonds indicated that carbonates could withstand the extreme pressures and temperatures of not only the upper mantle, but the lower mantle as well.
Previous research had shown that upper mantle carbonates are magnesium-rich and iron-poor. Under lower mantle conditions, it is thought that the arrangement of electrons in carbonate minerals changes under the pressure stress in such a way that iron may be significantly redistributed. However, accurate observations of lower mantle carbonates' chemical composition are not possible yet.
A research team - Carnegie's Sergey Lobanov and Alexander Goncharov, along with Konstantin Litasov of the Russian Academy of Science and Novosibirsk State University in Russia - focused on the high-pressure chemistry of a carbonate mineral called siderite, which is an iron carbonate, FeCO3, commonly found in hydrothermal vents. Their findings help resolve questions about the presence of iron-containing lower mantle carbonates, and are published by American Mineralogist.
Until recently the electron-arrangement change responsible for iron redistribution in the lower mantle had not been measured in the lab. It was previously discovered that this change, a phenomenon called a spin transition, took place between about 424,000 and 484,000 times normal atmospheric pressure (43 to 49 gigapascals).The team was able to pinpoint that spin transition was occurring in iron carbonates under about 434,000 times normal atmospheric pressure (44 gigapascals), typical of the lower mantle.
A spin transition is a rearrangement of electrons in a molecule or a mineral (figure 1 below). Electrons hold a compound's atoms together by bonding. Certain fundamental rules of chemistry govern this bonding process, which have to do with the energy it takes to form the bonds. Pressure-induced spin transitions rearrange electrons and change the energy of the chemical bonds. If the change in chemical bond energy is high enough, the spin transition may trigger iron redistribution between coexisting minerals.
To quantify the energy change, siderite's spin transition was examined using highly sensitive spectroscopic techniques at pressures ranging from zero to about 711,000 times normal atmospheric pressure (72 gigapascals), and also revealed by a visible color change after the transition, indicating rearrangement of electrons. The obtained spectroscopic data provided the key ingredient to estimating the carbonate composition at pressures exceeding the spin transition-pressure. It turned out that lower mantle carbonates should be iron-rich, unlike upper mantle carbonates (figure 2 below). Similar effects may exist in other lower mantle minerals, if they also undergo spin transitions.
"As we learn more about how the spin transition affects chemical composition in carbonates, we improve our understanding of all iron-bearing minerals, enhancing our knowledge about lower mantle chemistry," said Lobanov.
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.


























































