Nanostructures: customized carbon
A new synthesis method produces novel nanostructures from carbon
Advertisement
They are tiny and comprise spherical, sheet-like or fibrous particles. And they consist chiefly of the chemical element carbon. The talk here is about unusual carbon nanostructures that scientists at the Max Planck Institute of Colloids and Interfaces in Potsdam-Golm are now producing with a novel method. The researchers have already shown that their nanostructures possess useful catalytic properties: for instance, they can reduce the energy required to break down water by electrolysis. This is a useful property for storing renewable energy. And because such nanoparticles contain large porosity, the scientists believe that they could also conceivably be used to store gases, such as carbon dioxide and in further applications.
If you leave a pizza in the oven too long, the dough turns black. During the charring process, organic constituents in the dough are converted to species with high carbon content. Though the effect is undesirable in the kitchen, it is actually the chief aim of some industrial processes. One example of carbonisation is the conversion of coal into coke to increase the carbon content. Industrial soots, such as those used as pigments in car tyres, also have high carbon content thanks to controlled incomplete combustion.
For some years now scientists have been working on the controlled synthesis of carbon-rich nanomaterials. Because such particles are highly porous, have a large specific surface and in some cases are also good electrical conductors, they have many potential applications. Using common techniques, typically spherical particles are obtained. With the help of a new method, researchers at the Max Planck Institute of Colloids and Interfaces in Potsdam-Golm have now succeeded in producing not only spherical but also sheet-like and fibrous nanostructures.
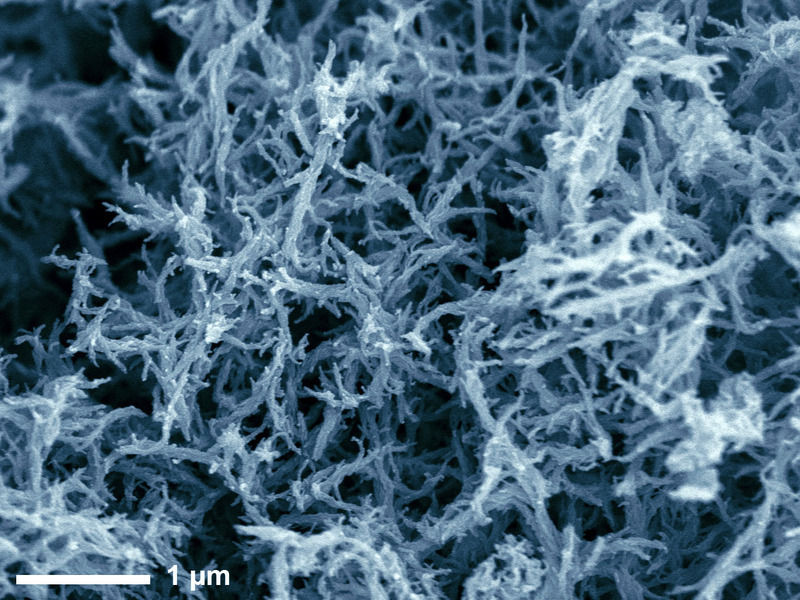
A recipe for nanofibres: Scientists at the Max Planck Institute of Colloids and Interfaces produce spherical, sheet-like and fibrous nanostructures by carbonising various organic solvents in hot salt melts. Until now, it was only possible to carbonise solids, resulting in spherical particles.
© MPI of Colloids and Interfaces
The starting substances determine the structure of the particles
The researchers started with a total of ten different organic solvents, each of which they then carbonised. “We found that we can control the spatial structure of the resulting particles by selecting suitable starting substances,” says Tim Fellinger, who leads the Carbon and Energy Group at the Potsdam-based Max Planck Institute.
Not only has his Group produced a variety of carbon nanostructures, they have also found ways to selectively introduce elements other than carbon into the products. For example, solvents containing nitrogen or sulphur, such as pyridine and dimethyl sulphoxide, result in nanostructures containing up to 15 percent nitrogen or sulphur by weight. By introducing suitable additives, the researchers were even able to incorporate metals such as nickel, cobalt and zinc to produce nanocomposites.
Nickel-carbon composites as catalysts for hydrolysis
Initial experiments with the nanostructured products have uncovered many useful properties. Because Fellinger’s Group is also exploring energy storage solutions, they are investigating the catalytic use of nanocarbons in the electrochemical hydrolysis of water. In this application, nickel-carbon nanocomposites in particular have proved to be as efficient as conventional catalysts. “But they’d probably be more economical to produce than the iridium-based catalysts commonly used today,” says Fellinger. Hydrolysis can be used, for example, to store excess electrical energy in the form of hydrogen for brief periods. “With cost-effective catalysts, the decentralised production of hydrogen on demand is also conceivable,” Fellinger adds. Risks carried with the transport of the gas would then be a thing of the past.
The scientists were impressed by how porous their nanostructures are and how well the carbon particles adsorb gases. Some products even adsorbed gases better than commercial activated charcoal, which was optimized for that purpose. Tim Fellinger finds that remarkable: unlike with active charcoal, no measures are taken during the carbonisation process to increase the adsorption capacity. Fellinger believes this opens up a wealth of potential applications. For example, the new particles might prove useful in the development of next-generation batteries, e.g. lithium-sulphur or lithium-air batteries.
A new synthesis pathway produces structural variety
Two approaches were key to achieving the structural variety and useful properties of the nanostructures, both of which were unexplored territory. First, the researchers carried out instant carbonization at high temperature in the liquid state. They used an uncommon reaction milieu of salt melts at over 500 degrees, for instance liquid zinc chloride. Second, they carbonised liquid starting substances. Previously, solids were mainly carbonised, because the high temperatures required would cause organic liquids to evaporate away. To this end, the researchers simply inject inexpensive off-the-shelf solvents into the liquid salt.
“Evidently, the liquid molecules dissociate upon contact with the melt, even before they can evaporate,” Tim Fellinger explains. “The dissociated products then presumably combine to form larger carbon-rich molecules within a few nanoseconds.” The zinc chloride melt appears to stabilise this process. Because salt melts are hot ionic fluids, chemists have coined the term ionothermal synthesis to describe syntheses in such milieus. These processes have already proved useful in inorganic chemistry. The Max Planck researchers in Potsdam are exploring it as carbonisation method.
After the reaction, they simply add dilute hydrochloric acid to the cooled mixture. While the salt in the mixture is dissolved by the acid, the nanocarbons – in the form of a black, fluffy powder – remain behind and are readily filtered out. Scanning electron microscopy is used to show the various nanostructures of the products obtained. For example, acetonitrile, benzonitrile and dimethyl sulphoxide gave rise to spherical products, as found in conventional industrial soots. However, dripping ethylene glycol or glycerol into the salt melt produces sheet-like particles. Other liquids such as ethanol, acetone and pyridine result in branched, interconnected fibrous products. The spherical carbon particles are ten nanometers in diameter, while the fibre-like structures are up to 120 nanometres long.
Salt melts act like lubricants and detergents
Although the precise mechanisms are still a matter of speculation, Tim Fellinger believes the new spectrum of particle structures to be entirely plausible: “We suspect that the salt melt acts as a kind of lubricant, increasing the mobility of the organic fragments.” This mobility, in turn, leads to more ways in which the building blocks can be arranged, he explains. The speed at which this occurs can differ from one solvent to the next, and this is one reason for the variety of structures. The chemist and nanostructure expert also thinks another factor is at work: “The salt reduces the surface tension.” This means that the carbon fragments no longer have to assume a spherical shape to minimise their surface area – just like water no longer forms drops on surfaces after detergent has been added.
The researchers also believe that salt ions are responsible for the impressive porosity of their nanocarbons: because of the low surface tension, the salt and carbon have large contact surfaces during synthesis. “After the salt is separated out, numerous pores remain,” Fellinger explains.
The researchers have a wealth of new ideas to explore. Given the large number of inorganic salts and organic solvents that can be combined with the new technique, there are likely to be many more customized composite variants with useful applications. The researchers now plan to experiment with other salt-solvent combinations. They also plan to investigate more closely whether the carbon sheets and fibres they discovered have advantages over spherical structures in specific applications. “In any case, we now have a new versatile carbonisation tool in the form of the hot injection of readily available solvents combined with ionothermal synthesis,” says Tim Fellinger.
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.






























































