Catalyst redefines rate limitations in ammonia production
Advertisement
Studies by researchers at Tokyo Institute of Technology have developed a catalyst that is so effective at promoting dissociation of the nitrogen bond in ammonia production reactions that it is no longer the step limiting the rate of the reaction.
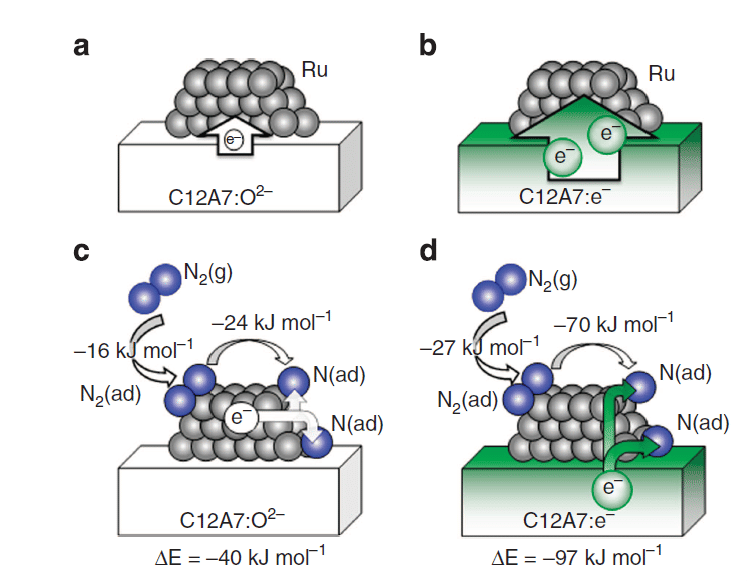
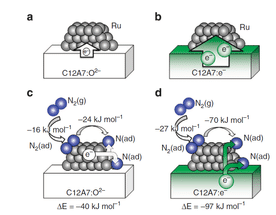
Ab initio simulations of N2 interaction with the Ru/C12A7 catalysts. Character of the charge redistribution between C12A7 substrate and deposited Ru clusters is shown for the stoichiometric (a) and the electride (b) C12A7. (c,d) Adsorption energies of N2 on C12A7-supported Ru, charge transfer in the process of N2 dissociation (N2(g)+ Ru → 2N(ad) +Ru) and the corresponding energy gain (ΔE). In Ru/C12A7:O2– system (c), N2 and N accept electron charge from the Ru cluster, making it positively charged. In Ru/C12A7:e– (d), the electron charge is transferred from the substrate, leaving the Ru cluster nearly neutral. N2(g), N2(ad) and N(ad) represent N2 in gas phase, adsorbed N2, and adsorbed nitrogen atom, respectively.
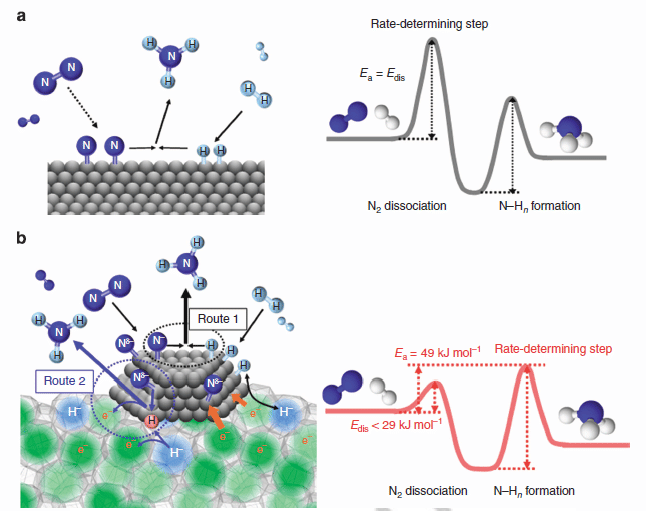
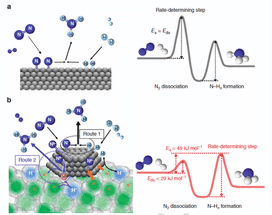
Proposed reaction mechanism and energy profile for ammonia synthesis. Reaction mechanism and energy profile for ammonia synthesis over (a) conventional catalyst and (b) Ru/C12A7:e-. (a) N2 and H2 react on the catalyst surface through a Langmuir–Hinshelwood mechanism to form NH3 in which N2 dissociation is the RDS. The energy barrier (Edis) for this step corresponds to the apparent activation energy (Ea) for ammonia synthesis. As for Ru/C12A7:e- (b), the rate-limiting step is not N2 dissociation but the formation of N–Hn species. NH3 is formed through the Langmuir–Hinshelwood mechanism (route 1) and the direct reaction of N adatoms with H radicals (nascent hydrogen) derived from cage H_ anions (route 2). Ea is determined by the difference between the top of the barrier for N–Hn formation and the energy level of reactant molecules (N2 and H2).
Ammonia (NH3) is crucial for the industrial synthesis of fertilizers and pharmaceuticals so that ways to improve its production from molecular nitrogen and hydrogen are in high demand. So far the main challenge has been breaking the triple bond in nitrogen molecules, which is the strongest bond in a molecule of two atoms. Now a collaboration of researchers in Japan, the UK and the US have developed a catalyst that is so effective towards the break of the nitrogen triple bond and found that this is no longer the rate-limiting step of the reaction.
Certain oxides can be very effective at enhancing the catalytic activity of ruthenium and iron but they are unstable in ammonia synthesis conditions. Recently the electride 12CaO·7Al2O3:e- (C12A7:e-) - an ionic compound with an electron acting as the negative ion – was found to be stable at room temperature. The discovery prompted Hideo Hosono and colleagues at Tokyo Institute of Technology and the Japan Science and Technology Agency in Japan, University College London in UK and Pacific Northwest National Laboratory in US to investigate Ru/C12A7:e- as a catalyst in ammonia production.
The researchers examined the N2 isotope exchange and hydrogen adsorption/desorption reactions. They observed a remarkable level of catalytic activity at less than half the activation energy of other catalysts. In addition the catalyst did not degrade due to hydrogen poisoning as is usually the case for ruthenium-based catalysts.
Further studies and density functional calculations suggested a mechanism for the reaction. “Fast N2 cleavage is ensured by highly efficient electron transfer from C12A7:e- to N2 molecules adsorbed on the Ru nanoparticles,” the researchers conclude in their report. “As a result, the bottleneck in the NH3 synthesis reaction is shifted from the N-N triple bond dissociation to the formation of nitrogen-hydrogen species.”



























































