Simple method of binding pollutants in water
New types of membrane adsorbers remove unwanted particles from water and also, at the same time, dissolved substances such as the hormonally active bis-phenol A or toxic lead. To do this, researchers at the Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB imbed selective adsorber particles in filtration membranes. From 24th to 27th March 2015, the IGB presents the membrane adsorbers and other innovative technologies for water treatment at the “Wasser Berlin International” Trade Fair and Congress. The Fraunhofer IGB is in Hall 2.2, Booth 422.
It was not until January 2015 that the European Food Safety Authority (EFSA) lowered the threshold value for bisphenol A in packaging. The hormonally active bulk chemical is among other things a basic material for polycarbonate from which, for example, CDs, plastic tableware or spectacles glasses are manufactured. Due to its chemical structure, bisphenol A is not completely degraded in the biological stages of treatment plants and is discharged into rivers and lakes by the purification facility.
Activated carbon or adsorber materials are already used to remove chemicals, anti-biotics or heavy metals from waste or process water. However, a disadvantage of these highly porous materials is the long contact time that the pollutants require to diffuse into the pores. So that as many of the harmful substances as possible are captured even in a shorter time, the treatment plants use larger quantities of adsorbers in correspondingly large treatment basins. However, activated carbon can only be regenerated with a high energy input, resulting for the most part in the need to dispose of large quantities of material contaminated with pollutants.
Also, membrane filtration with nanofiltration or reverse osmosis membranes, which can remove the contaminating substances, is not yet cost-effective for the removal of dissolved molecules from high-volume flows such as process or wastewater. Membranes filter the water through their pores when a pressure is built up on one side of the membrane, thus holding back larger molecules and solid particles. But the smaller the membrane pores are, the higher the pressure – and therefore the more energy – that is required to separate the substances from water.
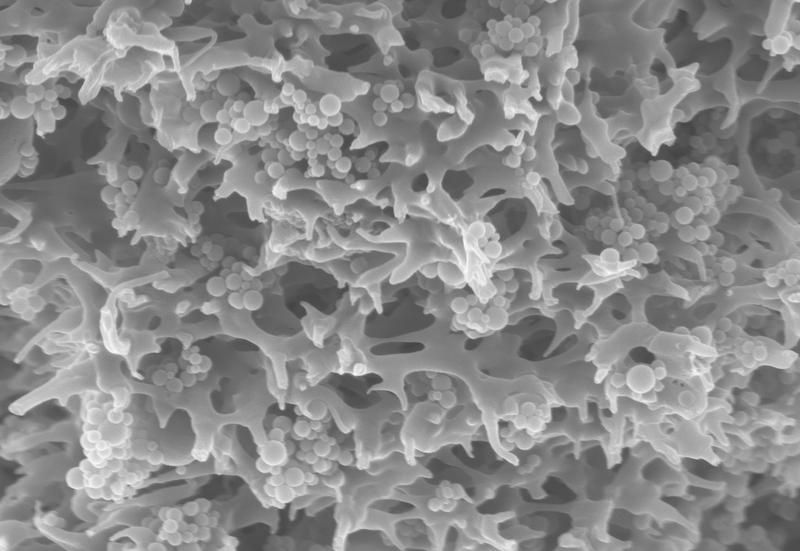
Tiny polymeric particles that bind pollutants from the water are imbedded in the porous carrier structure of the membrane adsorbers.
Fraunhofer IGB
Membrane adsorbers – filtering and binding in one step
Researchers at the Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB in Stuttgart have opted for a new approach that combines the advantages of both methods. When manufacturing the membranes they add small, polymeric adsorber particles. The resulting membrane adsorbers can – in addition to their filtration function – adsorptively bind substances dissolved in water. “We make use of the porous structure of the membrane located underneath the separation layer. The pores have a highly specific surface so that as many particles as possible can be imbedded, and they also provide optimum accessibility,” says Dr. Thomas Schiestel, Head of the “Inorganic Interfaces and Membranes” working group at the Fraunhofer IGB.
“Unlike conventional adsorbers, our membrane adsorbers transport the pollutants convectively. This means that, with the water flowing rapidly through the membrane pores, a contact time lasting only a few seconds is sufficient to adsorb pollutants on the particle surface,” says the scientist. Up to 40 percent of the weight of the membrane adsorbers is accounted for by the particles, so their binding capacity is correspondingly high. At the same time the membrane adsorbers can be operated at low pressures. As the membranes can be packed very tightly, very large volumes of water can be treated even with small devices.
Functional adsorber particles
The researchers manufacture the adsorber particles in a one-step, cost-efficient process. In this patented process monomeric components are polymerized with the help of a crosslinking agent to generate 50 to 500 nanometer polymer globules. “Depending on which substances are to be removed from the water, we select the most suitable one from a variety of monomers with differing functional groups,” Schiestel explains. The spectrum here ranges from pyridine, which tends to be hydrophobic, by way of cationic ammonium compounds and includes anionic phosphonates.
Selective removal of pollutants and metals
The researchers were able to show in various tests that the membrane adsorbers remove pollutants very selectively by means of the particles, which are customized for the particular contaminant in question. For example, membrane adsorbers with pyridine groups bind the hydrophobic bisphenol A especially well, whereas those with amino groups adsorb the negatively charged salt of the antibiotic penicillin G.
“The various adsorber particles can even be combined in one membrane. In this way we can remove several micropollutants simultaneously with just one membrane adsorber,” says Schiestel, pointing out a further advantage. Equipped with different functional groups, the membrane adsorbers can also remove toxic heavy metals such as lead or arsenic from the water. Phosphonate membrane adsorbers, for example, adsorb more than 5 grams of lead per square meter of membrane surface area – 40 percent more than a commercially available membrane adsorber.
Cost-effective and regenerable
So that the membrane adsorbers can be used several times, the adsorbed pollutants have to be detached once again from the particles in the membrane. “Membrane adsorbers for bisphenol A can be fully regenerated by a shift of the pH value,” Schiestel explains. The concentrated pollutants can then be disposed off cost-effectively or broken down using suitable oxidative processes.
The regenerability of the membrane adsorbers also makes possible a further application: reutilization of the separated molecules. This additionally makes the technology attractive for recovering valuable precious metals or rare earth metals.
Original publication
K. Niedergall, M. Bach, T. Hirth, G.E.M. Tovar, T. Schiestel (2014); "Removal of micropollutants from water by nanocomposite membrane adsorbers."; Sep. Purif. Technol. 131: 60-68.