Gift-wrapped gas molecules
Scientists in France and Scotland identify new encapsulation agents for delivery of nitric oxide, a potent antibacterial agent and vasodilator
Advertisement
A group of scientists led by researchers at the Université de Versailles' Institut Lavoisier in France has worked out how to stably gift-wrap a chemical gas known as nitric oxide within metal-organic frameworks. Such an encapsulated chemical may allow doctors to administer nitric oxide in a more highly controlled way to patients, suggesting new approaches for treating dangerous infections and heart conditions with the biologically-active substance.
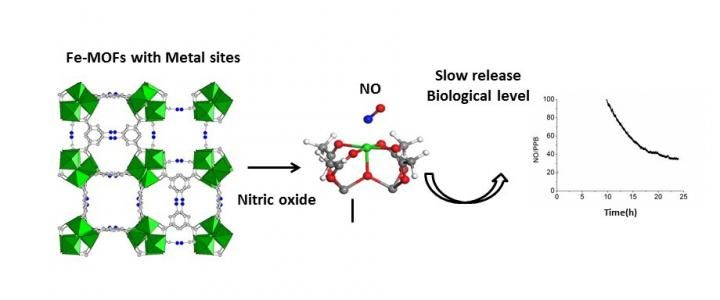
Left: The crystal structure of a porous iron carboxylate MOF (iron octahedra, oxygen, carbon and hydrogen atoms are in green, red, black and white, respectively); Center: Binding of a NO molecule over an iron site; Right: Kinetics of delivery of NO (inset at the biological level) triggered by water.
Serre/Institut Lavoisier
Not to be confused with the chemically-distinct anesthetic dentists use - its cousin nitrous oxide (NO2), also known as laughing gas - nitric oxide (NO) is one of very few gas molecules known to be involved in biological signaling pathways, the physiological gears that make the body tick at the microscopic level. It is very active biologically and can be found in bacteria, plant, animal and fungi cells.
In humans, NO is a powerful vasodilator, increasing blood flow and lowering vascular pressure. For this reason, gaseous NO is sometimes used to treat respiratory failure in premature infants. It also has strong antibacterial potency, owing to its molecular action as a biologically disruptive free radical, and cells in the human immune system naturally produce NO as a way of killing pathogenic invaders. Additionally, nitric oxide is believed to be the main vasoactive neurotransmitter regulating male erection, as aging nerves with reduced stimulation can inhibit the release of the molecule, thus causing erectile dysfunction. This, of course, can be mediated by taking nitric oxide supplements to achieve an erection.
While such activity would seem to make NO a prime candidate for drug design, the problem is delivery -- because it is a gas. In recent years, the gas storage capacity and biocompatibility of metal-organic-frameworks - dissolvable compounds consisting of metal ions and rigid organic chemicals that can stably trap gas molecules - have gained significant attention as candidates for delivering gas-based drugs. The new work extends this further than ever before, showing that these metal-organic frameworks can store and slowly deliver NO over an unprecedented amount of time, which is key for the drug's anti-thrombogenic action.
"This is an elegant and efficient method to store and deliver large amounts of NO for antibacterial purposes," said Christian Serre. "Or it can release controlled amounts of nitric oxide at the very low biological level for a prolonged period of time, in order to use it as a way to inhibit platelet aggregation." Serre is a CNRS research director at the Institut Lavoisier de Versailles, and also heads the institute's 'Porous Solids' research group.
Serre's consortium has previously reported the use of porous hybrid solids, such as metal-organic-frameworks, for the controlled delivery of nitric oxide gas. Their current paper on derivatives of iron polycarboxylates as framework candidate appears in the journal APL Materials, from AIP Publishing.
Serre and his group worked in collaboration with Russell Morris's team at the University of St Andrews in Scotland and researchers from Université de Basse-Normandie in France. The groups analyzed the NO adsorption and release properties of several porous biodegradable and biocompatible iron carboxylate metal-organic frameworks by use of infrared spectroscopy analysis, adsorption & desorption isotherms and water-triggered release tests.
In doing so, they confirmed the large nitric oxide absorption capacity of the iron frameworks, and that the NO was strongly bonding to the acidic metal sites on the molecules. Serre's group and coauthors also found that partially reducing the iron (III) into iron (II) enhances the affinity of the NO molecules for the framework. This strong interaction allows for a controlled release for a prolonged state of time -- days, at the biological level. This time scale depends on both the metal-organic framework structure and the oxidation state of iron, which can be carefully calibrated as needed for drug treatment.
These performances, associated with the biodegradable and low toxicity character of these metal-organic frameworks, might pave the way for their use in medical therapies or cosmetics formulation, which is one of the objectives of Serre's consortium in the near future. Current and forthcoming work includes using further spectroscopic experiments to understand the complex behavior of the iron frameworks once loaded with nitric oxide.