Thin film produces new chemistry in 'nanoreactor'
Physicists at the University of Groningen led by Professor of Functional nanomaterials Beatriz Noheda have discovered a new manganese compound that is produced by tension in the crystal structure of terbium manganese oxide. The technique they used to create this new material could open the way to new nanoscale circuits. Their findings were in Nature.
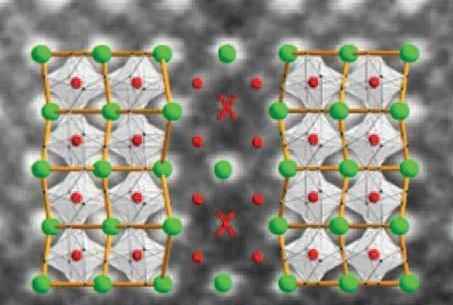
Two mirror-image domains (terbium in green, manganese in red, oxygen not shown) meet at a domain wall, where terbium atoms are squeezed out and replaced by manganese (red cross).
(c) Nature
The researchers grew a very thin layer (no more than a few dozen atoms thick) of the terbium manganese oxide crystal on a thicker base layer of strontium titanium oxide. This base layer affects the growth of the thin layer. When pieces of growing crystal meet, an interface or 'domain wall' develops, and the crystal structure comes under tensile stress in this wall.
Nanoreactor
Until a few years ago, materials scientists when creating very thin layers tried to prevent domain walls from occurring because of this tensile stress. 'Domain walls were seen as contamination', says Noheda. Then it became clear that the tension in the crystal structure actually invested the material with new properties, and, as has now become apparent, the domain wall can become a nanoscale chemical reactor.
Walls
The Groningen researchers have gained a lot of expertise in controlling how many domain walls develop. The composition of the base layer affects this, for instance, and the thinner the crystal layer, the greater the number of walls that occur.
'Alongside controlling how many walls develop, a further considerable challenge was to analyse exactly what happens in a wall, as this is generally only one atom thick', says Noheda. One way to analyse the material in the wall is to compare samples comprising different numbers of walls. The researchers saw that the more walls there were, the more magnetic the material was. 'Direct observation of a magnetic field is not yet possible on the atomic scale, particularly not in an isolator', says Noheda.
Zigzag line
An advanced atomic-resolution chemical analysis was used to show that the composition of the crystal in the walls had changed: in specific locations a manganese atom had taken the place of a larger terbium atom. The terbium atom forms a kind of zigzag line in the crystal structure. Two opposing zigzags meet in the domain wall, thus causing some of the terbium atoms to come into very close proximity. 'This creates significant tension, the terbium atom disappears from the crystal, and a smaller manganese atom takes its place', explains Noheda.
New chemistry
In contrast to the normal crystal, this extra manganese makes the wall magnetic. Professor of Theoretical Physics Maxim Mostovoy of the University of Groningen modelled the magnetism, and his results match the results of the experiment: 'A bond that has not yet been described occurs between five manganese atoms. We therefore see new chemistry in the domain wall.' This makes the domain wall a kind of nanoscale chemical reactor. 'And we suspect that this kind of new bond will occur in all crystals with this zigzag structure.'
Noheda hopes in further research to generate walls with the potential to form circuits. Minute circuits of only a few atoms in size could then develop. 'But I also hope that chemists will set to work on these nanoreactors.'
Original publication
S. Farokhipoor, C. Magén, S. Venkatesan, J. Íñiguez, C.J.M. Daumont, D. Rubi, E. Snoeck, M. Mostovoy, C. de Graaf, A. Müller, M. Döblinger, C. Scheu and B. Noheda; "Artificial chemical and magnetic structure at the domain walls of an epitaxial oxide."; Nature, 2014.
Original publication
S. Farokhipoor, C. Magén, S. Venkatesan, J. Íñiguez, C.J.M. Daumont, D. Rubi, E. Snoeck, M. Mostovoy, C. de Graaf, A. Müller, M. Döblinger, C. Scheu and B. Noheda; "Artificial chemical and magnetic structure at the domain walls of an epitaxial oxide."; Nature, 2014.
Organizations
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.