Synthesis of structurally pure carbon nanotubes using molecular seeds
For the first time, researchers at Empa and the Max Planck Institute for Solid State Research have succeeded in "growing" single-wall carbon nanotubes (CNT) with a single predefined structure - and hence with identical electronic properties. And here is how they pulled it off: the CNTs "assembled themselves", as it were, out of tailor-made organic precursor molecules on a platinum surface, as reported by the researchers in the latest issue of the journal "Nature". In future, CNTs of this kind may be used in ultra-sensitive light detectors and ultra-small transistors.
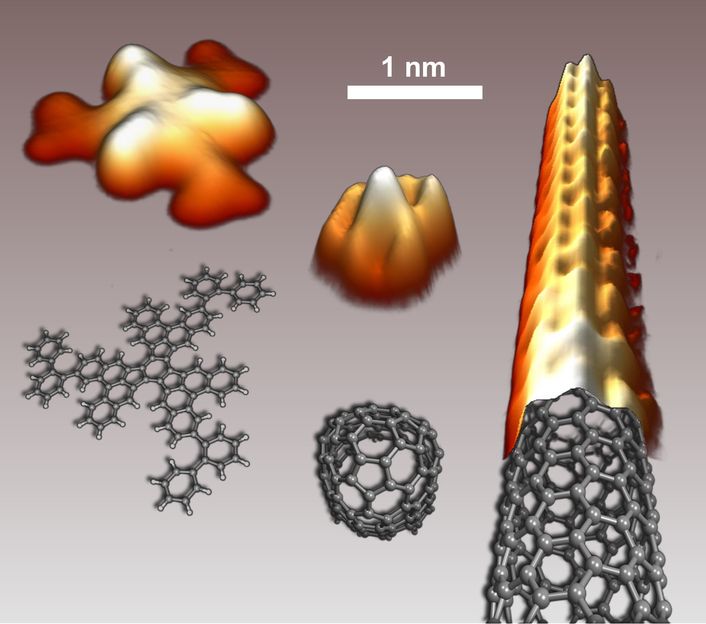
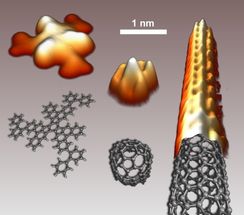
Scanning tunneling microscopy images the precursor, the «folded» end cap, and the resulting carbon nanotube, together with the corresponding structural models.
Empa / Juan Ramon Sanchez Valencia
On a Platinum surface, the planar hydrocarbon precursor folds into an end cap, that in turn acts as seed for the growth of a well-defined carbon nanotube. The image below shows scanning tunneling microscopy images of the precursor, the «folded» end cap, and the resulting carbon nanotube, together with the corresponding structural models.
Empa / Juan Ramon Sanchez-Valencia
For 20 years, carbon nanotubes (CNTs) have been the subject of intensive fundamental as well as applied research. With their extraordinary mechanical, thermal and electronic properties, these tiny tubes with their graphitic honeycomb lattice have become the paragon of nanomaterials. They could help to create next-generation electronic and electro-optical components that are smaller than ever before, and thus to achieve even faster switching times.
As uniform as possible
With a diameter of roughly one nanometre, single-wall CNTs (or SWCNTs) need to be considered as quantum structures; the slightest structural changes, such as differences in diameter or in the alignment of the atomic lattice, may result in dramatic changes to the electronic properties: one SWCNT may be metallic, whilst another one with a slightly different structure is a semiconductor. Hence, there is a great deal of interest in reliable methods of making SWCNTs as structurally uniform as possible. In fact, corresponding synthesis concepts were formulated about 15 years ago. However, it is only now that surface physicists at Empa and chemists at the Max Planck Institute have successfully implemented one of these ideas in the laboratory. In the latest issue of "Nature", they describe how, for the first time, it has been possible to “grow" structurally homogenous SWCNTs and, hence, managed to clearly define their electronic properties.
For some time, the Empa team working under the direction of Roman Fasel, Head of the "nanotech@surfaces" Laboratory at Empa and Professor of Chemistry and Biochemistry at the University of Berne, has been investigating the subject of "how molecules can be transformed or joined together to form complex nanostructures on a surface". For instance, by means of "bottom-up" synthesis, the Empa researchers managed to produce specific nanostructures such as defined chains of "buckyballs" (essentially, CNTs shrunk into ball form) or flat nanoribbons on gold substrates. "The great challenge was to find the suitable starting molecule that would also actually 'germinate' on a flat surface to form the correct seed," says Fasel, whose team has gained broad expertise in the field of molecular self-organisation over the years. Finally, their colleagues at the Max Planck Institute in Stuttgart successfully synthesised the suitable starting molecule, a hydrocarbon with no fewer than 150 atoms.
Molecular origami
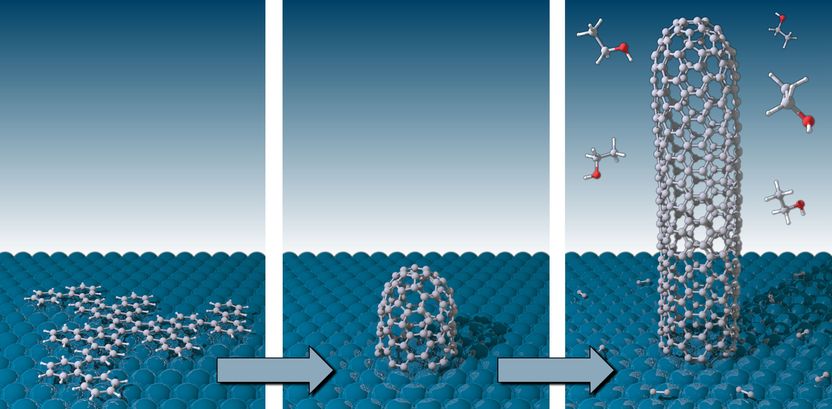
Now how does the process actually work? In the first step, in a manner reminiscent of origami, the flat starting molecule must be transformed into a three-dimensional object, the germling. This takes place on a hot platinum surface (Pt(111)) by means of a catalytic reaction in which hydrogen atoms are split off and new carbon-carbon bonds are formed at very specific locations. The "germ" – a small, dome-like entity with an open edge that sits on the platinum surface – is "folded" out of the flat molecule. This "end cap" forms the "lid" of the growing SWCNT. In a second chemical process, further carbon atoms are attached, which originate from the catalytic decomposition of ethylene (C2H4) on the platinum surface. They position themselves on the open edge between the platinum surface and the end cap and raise the cap higher and higher; the nanotube grows slowly upwards. Only the germ defines the latter's atomic structure, as the researchers were able to demonstrate through the analysis of the vibration modes of the SWCNTs and scanning tunnel microscope (STM) measurements. Further investigations using the new scanning helium ion microscope (SHIM) at Empa show that the resulting SWCNTs reach lengths in excess of 300 nanometres.
It works!
Thus the researchers have proved that, by using made-to-measure molecular "germs", it is possible to clearly predefine the growth (and thus the structure) of long SWCNTs. The SWCNTs synthesised in this study are mirror-image symmetrical entities. However, depending on the manner in which the honeycombed atomic lattice is derived from the starting molecule ("straight" or "oblique" in relation to the CNT axis), it would also possible be possible to produce helically-wound nanotubes, i.e. nanotubes twisting to the right or left, which are not mirror-image symmetrical. And this very structure also determines the electronic, thermoelectric and optical properties of the material. Therefore, in principle, the researchers can produce materials with different properties in a targeted manner, by selecting the starting molecule.
As their next step, Fasel and his colleagues intend to gain an even better understanding of the way in which SWCNTs populate a surface. Although well over 100 million nanotubes per square centimetre are already grown on the platinum surface, actual "fully-grown" nanotubes only grow from a comparatively small proportion of the germs. This raises the questions: which processes are responsible for this, and how can the yield be increased?