Free Pores for Molecule Transport
Researchers Identify Cause of Surface Barriers of Metal-Organic Frameworks (MOFs)
Metal-organic frameworks (MOFs) can take up gases similar to a sponge that soaks up liquids. Hence, these highly porous materials are suited for storing hydrogen or Greenhouse Gases. However, loading of many MOFs is inhibited by barriers. Scien-tists of Karlsruhe Institute of Technology (KIT) now report in “Nature Communications” that the barriers are caused by cor-rosion of the MOF surface. This can be prevented by water-free synthesis and storing strategies.
IFG/KIT
MOFs are crystalline materials consisting of metallic nodes and organic connection elements. They have a very large surface area and are highly porous. Like a sponge, they can take up other mole-cules. MOFs, produced on a large technical scale, are highly suited for the storage of gases: When the gas enters the solid, it is partly liquefied. The density increases and much more molecules can be stored in the same volume. Among others, MOFs are suited for the storage of hydrogen in the tank of hydrogen-driven automobiles. They can also be used for storing greenhouse gases like carbon dioxide and methane. Other applications are substance separation, catalysis, and sensor technology. For any application, an appropri-ate MOF can be produced. Mostly, MOFs have the form of a pow-der. In the past ten years, more than 20,000 different representatives of this class have been synthesized and characterized in detail.
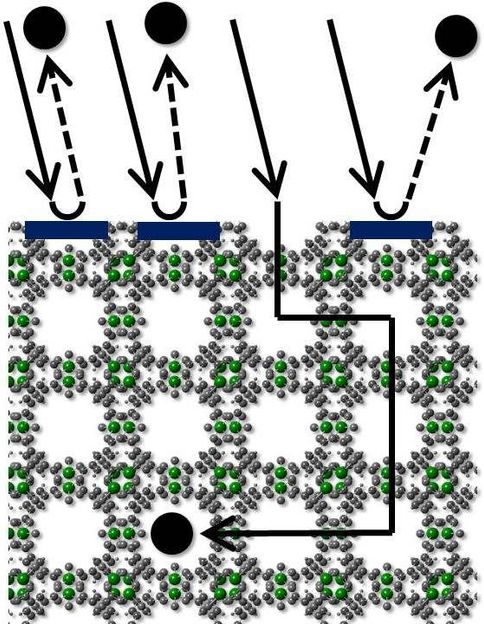
“For nearly all applications, loading of these highly porous crystals with molecules is essential,” Lars Heinke of the Institute of Function-al Interfaces (IFG) of KIT explains. “The efficiency of molecule transport into the porous particles is crucial to the performance of the MOFs.” In many MOF materials, however, loading is inhibited largely by so-called surface barriers. The surface of the sponge is broken, the pores are clogged, and loading is delayed significantly. This limits the application opportunities.
To better understand and identify the reasons of these problems, the IFG researchers studied the formation of surface barriers. For this purpose, they conducted fundamental experiments on thin, structurally perfect MOF layers mounted on solid substrates. These SURMOFs (SURface-mounted Metal-Organic Frameworks) are characterized by a high order and ideal structure. The researchers succeeded in attributing the barriers to a corrosion of MOF layers on the surface. They demonstrated how corrosion of the surface layer proceeds and found that water plays a central role. “Many scientists thought that these surface barriers are intrinsic and, hence, cannot be prevented. This assumption has now been disproved. It is possible to produce MOFs for loading without “clogging”,” says the Head of KIT’s IFG, Professor Christof Wöll. The work reported in the journal “Nature Communications” refutes several previous hypotheses.
The findings might be helpful for many applications of MOFs. Due to the results of the KIT researchers, water-free synthesis strategies for MOFs will have to be developed in the future. Improved materials will ensure barrier-free transport of molecules from the gas phase and liquid phase into MOFs. This will enable to further increase the efficiency of these promising storage and functional materials.
Original publication
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.



























































