Highly charged ions
Multiply-ionized atoms for clocks, qubits, and constants
Advertisement
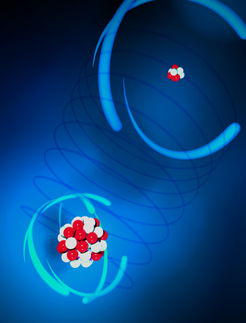
The world is mostly neutral. That is, most of the atoms in our environment are electrically neutral. The number of electrons in the outer parts of atoms equals the number of protons at the centers of atoms. As one or more electrons are plucked away from the atoms, the remaining electrons feel a much stronger positive pull from the nucleus. This enhanced pull, causing the atoms to shrink in size, ensures that those electrons are less vulnerable to the distractions of their environment, making them potentially valuable for next-generation atomic clocks, for quantum information schemes (where the loss of quantum coherence in qubits is a paramount danger), and for experiments trying to detect slight variations in the fine structure constant, the parameter that sets the overall strength of the electromagnetic force.
A new theoretical study conducted by JQI adjunct fellow Marianna Safronova and her colleagues from groups around the world provides the best yet study of how highly charged ions could be used for atomic timekeeping and for processing quantum information. They identify 10 such ions -for instance, samarium-14+ and neodymium-10+ - along with estimates of ion properties experimenters need to know before beginning their work, things such as the expected lifetimes and internal energy levels for the excited states of the ions.
Working with highly charged ions
Charged-up atoms are hard to produce and control. At one of the facilities dedicated to this purpose, the Electron Beam Ion Trap (EBIT) at the Lawrence Livermore National Lab, a beam of electrons intercepts a beam of atoms, ionizing the atoms at they go. In this way charge states all the way up to +92 (fully ionized uranium atoms) have been achieved. The trick is to store such charged ions and to cool them to low temperatures. In the kind of atomic environments typical of atomic clocks or quantum computers, low temperature means small ion motion, which makes for better spectroscopy.
Spectroscopy is all important for the applications mentioned above - clocks, quantum computing, and testing the constants of nature. Currently the world's time standard is pegged to a particular microwave transition in cesium-133 atoms.. Even higher precision and better clocks will result from the use of transitions in the optical range.
One problem of working with highly charged ions is that the gaps in the energy levels are too great. This is because when you ionize an atom, its energy levels get further apart. The light emanating from transitions in these ions is at too great a frequency, often in the ultraviolet part of the electromagnetic spectrum. Precision control and manipulation of ions needed for clocks and quantum information is harder or presently impossible to do in that UV or even x-ray energy regime. So in choosing ion candidates, it is important to search for transitions in the optical or near-optical range.
Another criterion is that the ions should be able to attain semi-stable excited states. A third criterion is that the characteristic transition is not between the states from the same electronic configuration. Such transitions do not have enhanced sensitivity to the study whether the fine structure constant is changing over time.. A fourth criterion is that the final ionic state should not be a radioactive substance, thus reducing handling problems.
Safronova and her colleagues, using these criteria and a state-of-the-art methods that they developed to study atoms arrived at their list of ten worthy ion species. They publish their results in Physical Review Letters.
Not the least part of their achievement is the authors' specification of the frequencies to be expected from the critical transitions in the candidate ions. It's hard enough to calculate the transition energies of un-ionized atoms, but even harder with these particular highly charged ions. They calculated the frequencies for their select ions and then compared (where appropriate) with the values observed in the lab. The difference, generally less than 1%, should reassure experimenters that Safronova and her colleagues can accurately predict properties of ions where no experimental data are available.
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!

Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!