Highly Reactive Gold Carbene Complex Shines in Emerald Green
Heidelberg chemists succeed in isolating carbon-gold compound of “amazing stability”
With a chemical “trick”, scientists at Heidelberg University have succeeded in isolating a stable gold carbene complex. Chemist Prof. Dr. Bernd F. Straub and his team are the first to have created the basis for directly examining the otherwise unstable gold-carbon double bond. Prof. Straub explains that highly reactive gold carbene molecules play an important role in landmark catalysing processes taking place at high speed. The research findings have been published in the German and the international edition of „Angewandte Chemie“.
Chemical reactions can be accelerated with the aid of catalysts; consequently materials and pharmaceuticals can be manufactured from the raw materials of nature. The study of gold compounds in catalytic processes has proved particularly intensive and successful, according to Prof. Straub. “In numerous scientific studies in the last ten years, experts have been proposing gold carbenes as essential short-lived intermediates in catalytic reactions,” the Heidelberg researcher explains. However, with their high reactivity they escape detailed study: hardly has a gold carbene fragment consisting of the elements gold and carbon emerged when it continues to react.
In order to first create a stable complex and isolate a gold carbene structure for research, the two elements were “lured into a cage like a hungry tiger with a bait,” says Matthias Hussong, who is working on his doctoral dissertation in Prof. Straub’s team. The researchers first shielded the gold and carbon from its environment by surrounding them with low-reactive, space-filling chemical groups. Then the two elements were bonded in a carefully planned step – and so the Au=C fragment was “caught” in the gold carbene complex.
The chemists were able to impart “an amazing stability” to the gold carbene, says Prof. Straub – and at the same time to make it literally visible. “Almost all gold complexes are colourless, while the ‘stable’ gold carbene is emerald green,” states the scientist, who heads a research group at Heidelberg University‘s Institute of Organic Chemistry. Further Heidelberg studies showed that gold in its compounds is more than a “soft proton”, as the chemical behaviour of gold had been described to date.
If the gold fragment is replaced by a “real” proton, e.g. the nucleus of hydrogen, the lightest element, this analogous protonated carbene displays a reddish purple colour. “The gold in the gold carbene complex behaves differently from a proton – that is very clear to the eye,” states Prof. Straub. He and his team are now continuing to explore the understanding of gold catalysis, with the aim of using these findings to make catalytic processes more efficient.
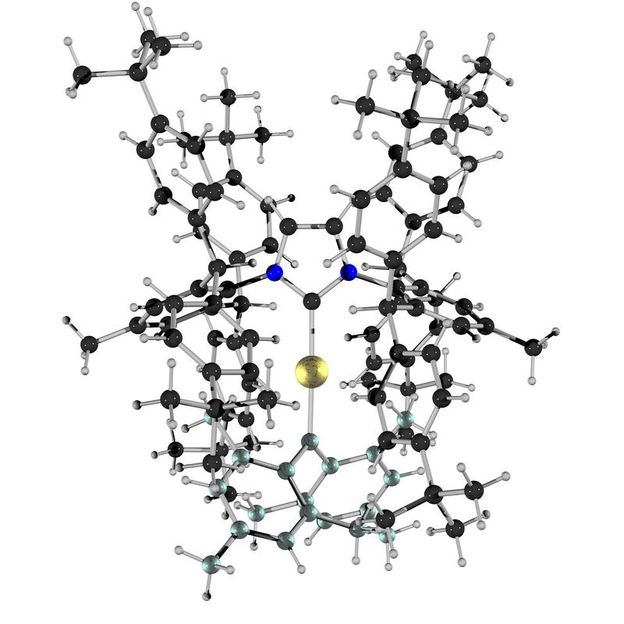
The Au=C double bond in the gold carbene compound is the bond between the large golden atom in the middle and the slightly greenish atom below.
Copyright: Matthias Hussong and Bernd F. Straub, Heidelberg University
Original publication
Most read news
Original publication
Hussong, M. W., Rominger, F., Krämer, P. und Straub, B. F.: "Isolierung eines nicht-Heteroatom-stabilisierten Goldcarbens."; Angew. Chem. 2014.
Hussong, M. W., Rominger, F., Krämer, P. and Straub, B. F.: "Isolation of a Non-Heteroatom-Stabilized Gold–Carbene Complex."; Angew. Chem. Int. Ed. 2014.
Organizations
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Better eco-efficiency of formates in gas drilling
Unlike rubber bands, molecular bonds may not break faster when pulled
Amine



























































