The behavior of charges in correlated spin-orbit coupled materials
Electronic disruption prods Mott insulator's conversion to metallic state
In a relatively recently discovered class of materials, known as spin-orbit Mott insulators, theorists have predicted the emergence of new properties at points just beyond the insulating state, when electronic manipulation can transform these compounds into conducting metals.
Nature Communications
A better understanding of electrons near this transition, theorists have predicted, could allow these new Mott insulators to pave the way to discoveries in superconductivity, new topological phases of matter, and new forms of unusual magnetism.
What scientists have lacked is experimental evidence that reveals the microscopic mechanisms that actually drive one of these spin-orbit Mott insulators to become a metal.
Now a team of physicists at Boston College report in Nature Communications that they manipulated a compound of strontium, iridium and oxygen – Sr3Ir207 – with a substitution of ruthenium metal ions, successfully driving the material into the metallic regime, and mapping this previously uncharted transformation as it took place, giving scientists a unique view into the workings of these insulators.
Spin-orbit Mott insulators are so named because of their complex electronic properties. Within these novel materials, there is a repulsive interaction between electrons that tends to drive the electrons to a stand still. This tendency is bolstered by the lowering of the electron's energy via a strong interaction between the electron's magnetic field and its orbital motion around the nucleus.
This delicate interplay between repulsive action, known as Coulomb interaction, and the coupling between electrons' spin and orbital motion has allowed scientists to define this class of materials as spin-orbit Mott insulators.
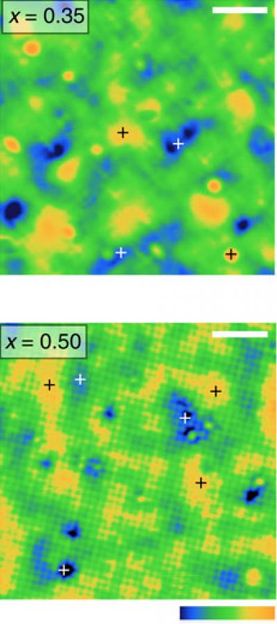
Boston College Assistant Professor of Physics Stephen D. Wilson said the team succeeded in driving the insulator-to-metal transformation by replacing 40 percent of the iridium ions with ruthenium, thereby creating a metal alloy. That event introduced charge carriers, which have proven successful in destabilizing the so-called Mott phase in the transformation of compounds in this class of insulators.
Scanning tunneling microscopy revealed ruthenium effectively created features within the compound that resembled minute metallic puddles, said Wilson, one of the lead researchers on the project. As the amount of additional ruthenium was increased, the puddles began to "percolate," coalescing to form a metal across which charges freely flow, he added.
"The addition of ruthenium introduces charge carriers, but at a low ratio of ruthenium to iridium they simply stay put in these little metallic puddles, which are symptoms of strong correlated electrons," Wilson said. "These electrons are stable and wouldn't move much. But when we stepped up the disruption by increasing the amount of ruthenium, the puddles moved together and achieved a metallic state."
The behavior in this particular compound parallels what researchers have seen in Mott insulators that play host to such phenomenon as high temperature superconductivity, said Wilson.
By pinpointing exactly where this transformation takes place, the team's findings should help to lay the groundwork in the scientific search for new electronic phases within spin-orbit Mott insulators, said Wilson, who co-authored the report with his Boston College Department of Physics colleagues Professor Vidya Madhavan, Professor Ziqiang Wang, and Assoc. Prof. Fr. Cyril P. Opeil, SJ.
Topics
Organizations
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Cholinesterase_enzyme
Three shows, one date - analytica Anacon India, India Lab Expo and PHARMA Pro & Pack Expo to collocate