Swiss cheese crystal, or high-tech sponge?
The sponges of the future will do more than clean house.
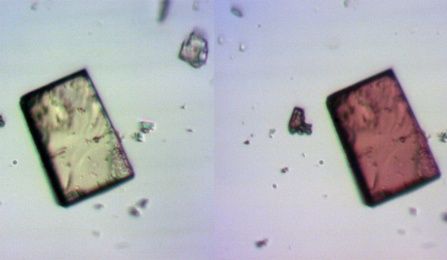
Created by chemists at the University at Buffalo and Penn State Hazleton, this sponge-like crystal contains many pores that change shape when exposed to ultraviolet (UV) light. In addition, the normally colorless crystal (left) blushes in the presence of UV light, turning red (right).
Ian M. Walton
Picture this, for example: Doctors use a tiny sponge to soak up a drug and deliver it directly to a tumor. Chemists at a manufacturing plant use another to trap and store unwanted gases.
These technologies are what University at Buffalo Assistant Professor of Chemistry Jason Benedict, PhD, had in mind when he led the design of a new material called UBMOF-1. The material — a metal-organic framework, or “MOF” — is a hole-filled crystal that could act as a sponge, capturing molecules of specific sizes and shapes in its pores.
Swiss cheese-like MOFs are not new, but Benedict’s has a couple of remarkable qualities:
- The crystal’s pores change shape when hit by ultraviolet light. This is important because changing the pore structure is one way to control which compounds can enter or exit the pores. You could, for instance, soak up a chemical and then alter the pore size to prevent it from escaping. Secure storage is useful in applications like drug delivery, where “you don’t want the chemicals to come out until they get where they need to be,” Benedict says.
- The crystal also changes color in response to ultraviolet light, going from colorless to red. This suggests that the material’s electronic properties are shifting, which could affect the types of chemical compounds that are attracted into the pores.
Benedict’s team reported on the creation of the UBMOF in the journal Chemical Communications. The paper’s coauthors include chemists from UB and Penn State Hazleton.
“MOFs are like molecular sponges — they’re crystals that have pores,” Benedict said.
“Typically, they are these passive materials: They’re static. You synthesize them, and that’s the end of the road,” he added. “What we’re trying to do is to take these passive materials and make them active, so that when you apply a stimulus like light, you can make them change their chemical properties, including the shape of their pores.”
Benedict is a member of UB’s New York State Center of Excellence in Materials Informatics, which the university launched in 2012 to advance the study of new materials that could improve life for future generations.
To force UBMOF-1 respond to ultraviolet light, Benedict and colleagues used some clever synthetic chemistry.
MOF crystals are made from two types of parts — metal nodes and organic rods — and the researchers attached a light-responsive chemical group called a diarylethene to the organic component of their material.
Diarylethene is special because it houses a ring of atoms that is normally open but shuts when exposed to ultraviolet light.
In the UBMOF, the diarylethene borders the crystal’s pores, which means the pores change shape when the diarylethene does.
The next step in the research is to determine how, exactly, the structure of the holes is changing, and to see if there’s a way to get the holes to revert to their original shape.
Rods containing diarylethene can be forced back into the “open” configuration with white light, but this tactic only works when the rods are alone. Once they’re inserted into the crystal, the diarylethene rings stay stubbornly closed in the presence of white light.
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.