Salty surprise: Ordinary table salt turns into 'forbidden' forms
High-pressure X-ray experiments violate textbook rules of chemistry
High-pressure experiments with ordinary table salt have produced new chemical compounds that should not exist according to the textbook rules of chemistry. The study at DESY's X-ray source PETRA III and at other research centres could pave the way to a more universal understanding of chemistry and to novel applications, as the international research team, led by Prof. Artem Oganov of Stony Brook University (State University of New York) and Prof. Alexander Goncharov of Carnegie Institution, report in the scientific journal Science.
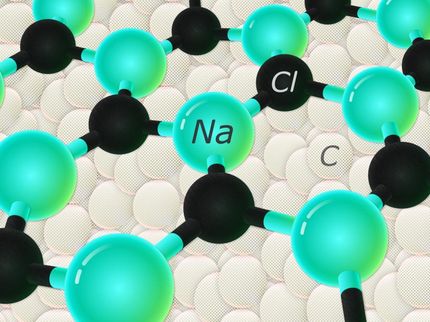
This is the structure of NaCl3.
Artem Oganov/Stony Brook University
Table salt, also known as sodium chloride or NaCl, is one of the best-known and most studied chemical compounds. It crystalises in a cubic unit cell and is very stable. Its chemical composition is simple - one sodium atom (Na) and one chlorine atom (Cl). Or at least that's true under ambient conditions. Other compounds of the two elements are forbidden by the classical rules of chemistry. For instance, according to the octet rule all chemical elements strive to fill their outermost shell with eight electrons, which is the most stable configuration, found in noble gases. Sodium has one extra electron and chlorine is missing one, so sodium donates one electron to chlorine, leaving both atoms with an outer shell containing eight electrons and forming a strong ionic bond.
But when the scientists put table salt under high pressure of 200,000 atmospheres and more at PETRA III and added an extra dash of either sodium or chlorine, "forbidden" compounds like Na3Cl and NaCl3 turned up. "Following the theoretical prediction, we heated the samples under pressure with lasers for a while," explains co-author Dr. Zuzana Konôpková of DESY, who supported the experiments at DESY's Extreme Conditions Beamline P02 (ECB). "We found other stable compounds of Na and Cl which came as a surprise." This is not supposed to happen, as these compounds require a completely different form of chemical bonding with higher energy, and nature always favours the lowest state of energy.
But Oganov's team had calculated before that exotic compounds might form under extreme conditions and remain stable under these conditions. "We have predicted and made crazy compounds that violate textbook rules: NaCl3, NaCl7, Na3Cl2, Na2Cl, and Na3Cl," says Dr. Weiwei Zhang, the lead author of the paper and a visiting scholar at Oganov's lab at Stony Brook. At PETRA III and at Carnegie Institution the scientists tested the predictions in what they call "cook and look" experiments, targeting Na3Cl and NaCl3, the two compounds that were predicted to be more easily made than others, and indeed found them. "These compounds are thermodynamically stable and once made, remain so indefinitely," says Zhang. "Classical chemistry forbids their very existence. Classical chemistry also says atoms try to fulfil the octet rule - elements gain or lose electrons to attain an electron configuration of the nearest noble gas, with complete outer electron shells that make them very stable. Well, here that rule is not satisfied."
The experiments help to explore a broader view of chemistry. "I think this work is the beginning of a revolution in chemistry," Oganov says. "We found, at low pressures achievable in the lab, perfectly stable compounds that contradict the classical rules of chemistry. If you apply rather modest pressure, 200,000 atmospheres – for comparison purposes, the pressure at the centre of the Earth is 3.6 million atmospheres – much of what we know from chemistry textbooks falls apart."
One reason for the surprising discovery is that textbook chemistry usually applies to what we call ambient conditions. "Here on the surface of the earth, these conditions might be default, but they are rather special if you look at the universe as a whole," Konôpková explains. What may be "forbidden" under ambient conditions on earth, can become possible under more extreme conditions. "'Impossible' really means that the energy is going to be high," Oganov says. "The rules of chemistry are not like mathematical theorems, which cannot be broken. The rules of chemistry can be broken, because impossible means softly impossible. You just need to find the conditions where the energy balance shifts and the rules hold no more."
Apart from its fundamental meaning, the discovery can also produce new practical applications. "When you change the theoretical underpinnings of chemistry, that's a big deal," Goncharov says. "But what it also means is that we can make new materials with exotic properties." Among the compounds Oganov and his team created are two-dimensional metals, where electricity is conducted along the layers of the structure. "One of these materials – Na3Cl – has a fascinating structure," Oganov says. "It is comprised of layers of NaCl and layers of pure sodium. The NaCl layers act as insulators; the pure sodium layers conduct electricity. Systems with two-dimensional electrical conductivity have attracted a lot interest."
The experiments with table salt might only be the beginning of the discovery of completely new compounds. "If this simple system is capable of turning into such a diverse array of compounds under high-pressure conditions, then others likely are, too," Goncharov explains. "This could help answer outstanding questions about early planetary cores, as well as to create new materials with practical uses."