New catalyst for cleanup of nitrites
Gold-palladium nanocatalysts set new mark for breakdown of nitrites
Advertisement
Chemical engineers at Rice University have found a new catalyst that can rapidly break down nitrites, a common and harmful contaminant in drinking water that often results from overuse of agricultural fertilizers.
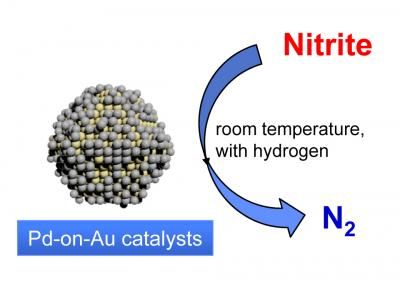
Researchers at Rice University's Catalysis and Nanomaterials Laboratory have found that gold and palladium nanoparticles can rapidly break down nitrites.
M.S. Wong/Rice University
Nitrites and their more abundant cousins, nitrates, are inorganic compounds that are often found in both groundwater and surface water. The compounds are a health hazard, and the Environmental Protection Agency places strict limits on the amount of nitrates and nitrites in drinking water. While it's possible to remove nitrates and nitrites from water with filters and resins, the process can be prohibitively expensive.
"This is a big problem, particularly for agricultural communities, and there aren't really any good options for dealing with it," said Michael Wong, professor of chemical and biomolecular engineering at Rice and the lead researcher on the new study. "Our group has studied engineered gold and palladium nanocatalysts for several years. We've tested these against chlorinated solvents for almost a decade, and in looking for other potential uses for these we stumbled onto some studies about palladium catalysts being used to treat nitrates and nitrites; so we decided to do a comparison."
Catalysts are the matchmakers of the molecular world: They cause other compounds to react with one another, often by bringing them into close proximity, but the catalysts are not consumed by the reaction.
In a paper in the journal Nanoscale, Wong's team showed that engineered nanoparticles of gold and palladium were several times more efficient at breaking down nitrites than any previously studied catalysts. The particles, which were invented at Wong's Catalysis and Nanomaterials Laboratory, consist of a solid gold core that's partially covered with palladium.
Over the past decade, Wong's team has found these gold-palladium composites have faster reaction times for breaking down chlorinated pollutants than do any other known catalysts. He said the same proved true for nitrites, for reasons that are still unknown.
"There's no chlorine in these compounds, so the chemistry is completely different," Wong said. "It's not yet clear how the gold and palladium work together to boost the reaction time in nitrites and why reaction efficiency spiked when the nanoparticles had about 80 percent palladium coverage. We have several hypotheses we are testing out now. "
He said that gold-palladium nanocatalysts with the optimal formulation were about 15 times more efficient at breaking down nitrites than were pure palladium nanocatalysts, and about 7 1/2 times more efficient than catalysts made of palladium and aluminum oxide.
Wong said he can envision using the gold-palladium catalysts in a small filtration unit that could be attached to a water tap, but only if the team finds a similarly efficient catalyst for breaking down nitrates, which are even more abundant pollutants than nitrites.
"Nitrites form wherever you have nitrates, which are really the root of the problem," Wong said. "We're actively studying a number of candidates for degrading nitrates now, and we have some positive leads."