Analysing interacting nuclear spins in solvents
Detection of long-range effects changes interpretation of NMR spectra
Advertisement
The RUB’s excellence cluster RESOLV has achieved successful results only a few months after its inception: by applying nuclear magnetic resonance spectroscopy, an international team comprised of researchers from the RUB and from the University of Vienna has solved a long-standing theoretical problem regarding the identification of solvent structures. Their model postulates that the coupling of nuclear spins in different molecules is determined by the frequency of the applied electromagnetic radiation. RESOLV strongly focuses on gaining an understanding of the solvents’ molecular characteristics.
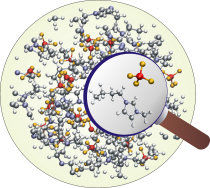
The image shows a computer simulation of a frequently-used ionic solvent. NOE experiments at low frequencies capture the structure of the entire area. Experiments at high frequencies provide information regarding the local structure of two neighbouring particles, highlighted by the magnifying glass.
© AG „Physikalische Chemie der Flüssigkeiten“
Overhauser
Hardly a spectroscopic method boasts so many different applications as nuclear magnetic resonance spectroscopy, better known as “NMR”. The approach of NMR spectroscopy is based on monitoring the so-called nuclear spin, namely the angular momentum of atoms, or, more precisely: the magnetic moment associated with them. The atom thus becomes a bar magnet, whose axis is aligned within a magnetic field but is otherwise arbitrarily oriented within its environment. The alignment can be altered by applying electromagnetic radiation in the radio wavelength, typically at several 100 Megahertz. The result is an NMR spectrum that is determined by the atom’s environment and provides a detailed insight into the structures and motions of molecules. NMR spectroscopy applications range from identifying molecules in chemical analysis to characterising large molecular clusters. Physicians, too, make use of NMR to look into the body, with CAT scan technology being the result of an NMR experiment. Among the various available methods, it is the Nuclear Overhauser Effect (NOE) analysis that is of particular importance. Anticipated by Albert Overhauser in 1953, this effect predicts a coupling of nuclear spins through interaction of their magnetic moments. Within the very same molecule this effect is extremely short-range and should apply only to neighbouring nuclear spins. On the basis of these postulations, a group of scientists headed by the Swiss chemist Kurt Wüthrich had developed methods for the identification of protein structures in solvents, for which he was awarded the Nobel Prize in Chemistry in 2002.
Interactions between the molecules
In proteins, the interacting nuclear spins belong to the same molecule; the fact that their relative distance does not vary in the course of the experiment facilitates their theoretical description. If the approach is extended to include monitoring of the interactions between molecules, it has the potential for various applications, for example the identification of the water structures and water dynamics on the surface of proteins or of local structures in novel “designer solvents” (so-called ionic fluids) –both of which are central issues for RESOLV. In these cases, the nuclear spins belong to different molecules and can move relatively to each other; the distance between them does not remain constant. This fact has made theoretical research difficult, and only partial solutions have been achieved to date. “A new theory regarding this effect has given this field of study a boost,” says Prof Dr Hermann Weingärtner, head of the RUB task force “Physical Chemistry of Fluids”. He has developed the theory in collaboration with Prof Dr Othmar Steinhauser (Institute for Computational Biological Chemistry at the University of Vienna). The team has succeeded in developing a realistic model in which all crucial factors are taken into consideration and which is manageable on a mathematical level. The results are surprising, because it turns out that the range of the Nuclear Overhauser Effect with spins in different molecules is determined by the frequency of the deployed electromagnetic radiation. Under typical experimental conditions, the effect is a long-range one. This, in turn, means that the experiments describe the structure of the entire fluid rather than just – as previously assumed – a molecule’s closest environment. The new theory thus constitutes the first step towards clarifying questions that have remained unanswered for a long time as well as conflicting interpretations of such experiments. Weingärtner and Steinhauser’s research has been financed by the “Cluster of Excellence RESOLV” (EXC 1069) of the German Research Foundation (DFG).
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!

Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!