New materials for bio-based hydrogen synthesis
Synthetic biology enables spontaneous protein activation
Advertisement
Researchers at the Ruhr-Universität Bochum (RUB) have discovered an efficient process for hydrogen biocatalysis. They developed semi-synthetic hydrogenases, hydrogen-generating enzymes, by adding the protein's biological precursor to a chemically synthesized inactive iron complex. From these two components, the biological catalyst formed spontaneously in a test tube. “Extracting hydrogenases from living cells is highly difficult,” says Prof Dr Thomas Happe, head of the work group Photobiotechnology at the RUB. “Therefore, their industrial application has always been a long way off. Now, we have made a decisive step towards the generation of bio-based materials.”
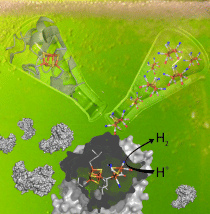
Activating hydrogen-generating enzymes: Once an inactive hydrogenase precursor (detail of the active centre: top left in the picture) is added to the inactive chemically synthesised cofactor (top right), the hydrogenase spontaneously integrates the synthetic component. Hydrogenases (bottom) are thus created that are indistinguishable from natural enzymes. The complex active centre of the enzymes (bottom middle) into which the cofactor is integrated catalyses the generation of hydrogen highly efficiently.
© Julian Esselborn, RUB
Application of hydrogenases: huge potential, difficult implementation
“Under ideal conditions, one single hydrogenase enzyme can generate 9,000 hydrogen molecules per second,” says Thomas Happe. “Nature has created a catalyst that is incredibly active even without any rare noble metals.” The researchers from Bochum examine so-called iron-iron [FeFe] hydrogenases whose catalysis is based on an active centre with a complex structure that contains iron, carbon monoxide and cyanide – only few living organisms are able to synthesize it. In order to skip the tedious and inefficient process of hydrogenase production, chemists have recreated the enzyme component that is catalytically active. Even though the reproduction was successful, these so-called mimics – chemical imitations – only generate small volumes of hydrogen (H2). Due to the difficulty of extracting active hydrogenases from living organisms, Thomas Happe's team suggested an optimisation of the method that had been reported by the research team from Bochum and their collaboration partners in “Nature” in June 2013.
Synthetic component enables the generation of H2 “at the push of a button”
The RUB biologists mixed the inactive hydrogenase precursor and the inactive chemical mimic, which was synthesised by their colleagues from Grenoble, within a test tube. A few minutes later, a strong generation of H2 was observed. The hydrogenase precursor had spontaneously integrated the chemically synthesised iron substance into its protein scaffold. Biophysical analyses at the MPI in Mülheim showed that the enzyme thus generated is indistinguishable from natural hydrogenase. “Until now, it has been assumed that enzymes with a complex structure such as hydrogenases require helper proteins to integrate the metal catalyst unit,” explains Happe. “When I proposed the idea for this experiment for the first time, nobody believed that it could work.”
Use of established commercial processes possible thanks to new method
“Mimics have made the process of working with hydrogenases much easier,” sums up Happe's PhD student Julian Esselborn. “Using the 'biotechnologist's pet' Escherichia coli, we are able to quickly produce several milligram of the hydrogenase's precursor. Subsequently, we add the chemical mimic and thereby create fully activated enzymes within a short period of time.” Industrial application is now within reach, because commercial processes for the cultivation of E. coli are already established. “The new method has the potential of becoming a milestone in hydrogenase research,” says Happe. It works with the hydrogenases of various organisms. “Moreover, it is suitable for high-throughput analysis of hydrogenase proteins that have been newly discovered or altered on the molecular biological level as well as of various – potentially optimised – chemical substances,” adds Julian Esselborn.
Hydrogen – clean energy carrier
Hydrogenases play an important role in the energy balance of many single-cell organisms. Their relevance for humans derives from the fact that they can help to generate a clean energy carrier, because hydrogen combusts to form pure water. Consequently, biologists and chemists have for many years been working towards making these enzymes and their chemical blueprints fit for industrial applications – as affordable and eco-friendly material for novel fuel cells and even for the direct generation of hydrogen from solar energy by means of photosynthesis.






























































