Tracing photochemical reactions
When light hits organic molecules, it triggers processes that are of considerable interest to scientists. But the individual steps of the reaction are very hard to identify. A study group at the University of Würzburg has now accomplished this task – with a sophisticated approach.
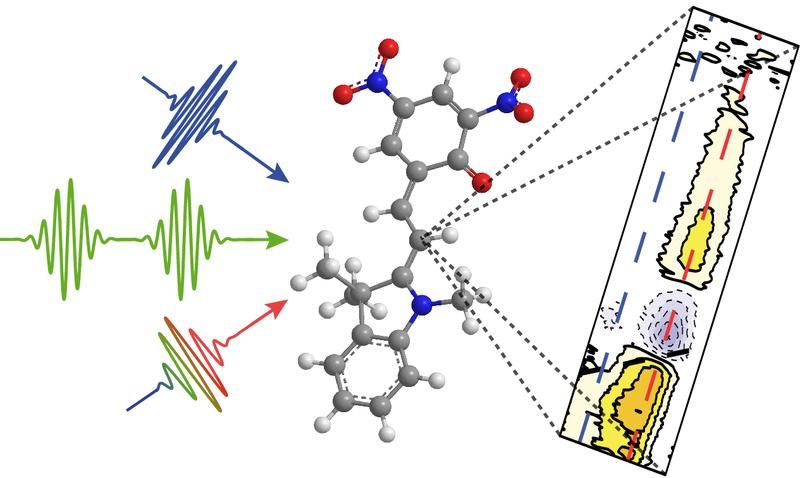
A sequence of ultrashort laser pulses (left) leads to the chemical reaction of a merocyanine dye (in the middle), which can be analyzed with multidimensional spectroscopy (right).
Martin Kullmann, University of Würzburg
It might well be every chemist's dream: to actively control chemical reactions at a molecular level, to form or break chemical bonds at will so as to create tailored substances with special properties. As a precondition, however, this requires highly accurate knowledge of the numerous individual steps of which chemical reactions usually consist. In many areas, such knowledge is not yet available and neither is it easy to gain.
But now Stefan Rützel and some of his colleagues in the team of Professor Tobias Brixner, the head of the Department for Physical Chemistry I at the University of Würzburg, have developed a method that can be used to clearly identify at least the precursor states of chemical reactions.
Research on a femtosecond time scale
There are two requirements for unlocking the secret of chemical reactions on an atomic scale: Speed and skill. This is because photochemical reactions are inconceivably fast even though they often include the formation of several intermediary products, usually taking place within the space of only a few femtoseconds, i.e. a few millionths of one billionth of a second.
Nevertheless, it is possible for the scientists to shed "light" on the chemical processes, using ultrashort laser pulses emitted by femtosecond lasers. The molecules are sort of "scanned" with the laser light over a certain time period so that the dynamics of the reaction processes can be mapped. This widely used method is known as "pump probe spectroscopy".
A laser pulse in duplicate
"Pump probe spectroscopy uses a laser pulse to initiate a certain reaction. A second laser pulse then probes the dynamics induced by the first pulse," Tobias Brixner says, explaining how the method works. This enables you, among other things, to determine the characteristic lifetime of excited states and to identify competing reaction pathways.
However, this method still does not solve the following problem: "In a pump probe experiment, it is very difficult to identify the special state of a molecule from where the reaction starts," Brixner explains. This is because the laser pulse creates a multitude of such states.
Ingenious experimental setup
Despite these difficulties, Stefan Rützel and some of his colleagues in Brixner's study group have now succeeded in clearly identifying such precursor states of reactions, using an ingenious experimental setup. For this purpose, they combined laser pulses of various wavelengths in the visible range with each other and studied their time-resolved correlation. In this way, they obtained information as to whether certain electronic transitions in the start and end states are quantum-mechanically linked to each other. In other words: Whether a certain electronic state is the precursor of another one.
In the experiment, the study group examined the molecule merocyanine, which exists in two distinct spatial arrangements, called conformations. After excitation with light, only one configuration gives rise to the formation of a cation, i.e. an ion with a positive charge, as the scientists were able to show. The method they developed thus enabled them to identify the special precursor that needs to be excited for the desired reaction to take place.
Promising method for application in photovoltaics and data processing
The researchers hope that this method of tracing reaction paths via electronic states might also be applied to the study of many other chemical processes. Potential areas of application include photovoltaic processes or data storage and data manipulation in optical storage media.
Original publication
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Pharmacies_of_Norway
Cut_(gems)

Synthetic leather made from recyclable and bio-based PBS
LANXESS to produce first bio-based EPDM rubber in the world
BASF increases capacity for waxes
Laser pulses control single electrons in complex molecules
Pyridostigmine
Breakthrough in molecular machines - Researchers have succeeded to obtain control of the molecular machines, which in the future may enable them to perform controlled movements
Anabolic_steroid
Bachem 2004 results with positive outlook for 2005
Tris(ethylenediamine)cobalt(III)_chloride