Small droplets grow differently
Initially, there is an unexpected growth spurt when droplets grow from moisture condensing on a surface
Advertisement
Fine dew drops on spider webs, blades of grass, and even insects can lend them breathtaking beauty. And, examining them very closely, one recognises that the drops themselves form astonishingly regular and aesthetic patterns. For the first time, scientists at the Max Planck Institute for Dynamics and Self-Organisation in Göttingen have now comprehensively investigated what laws these drops obey when they originate and grow in size. Elaborate computer simulations and experiments show that in particular the beginning of this growth phase proceeds differently than previously thought: the smallest droplets grow notably faster compared to their larger siblings. This new knowledge is especially important for irrigation technology and refrigeration.
© MPI for Dynamics and Self-Organisation

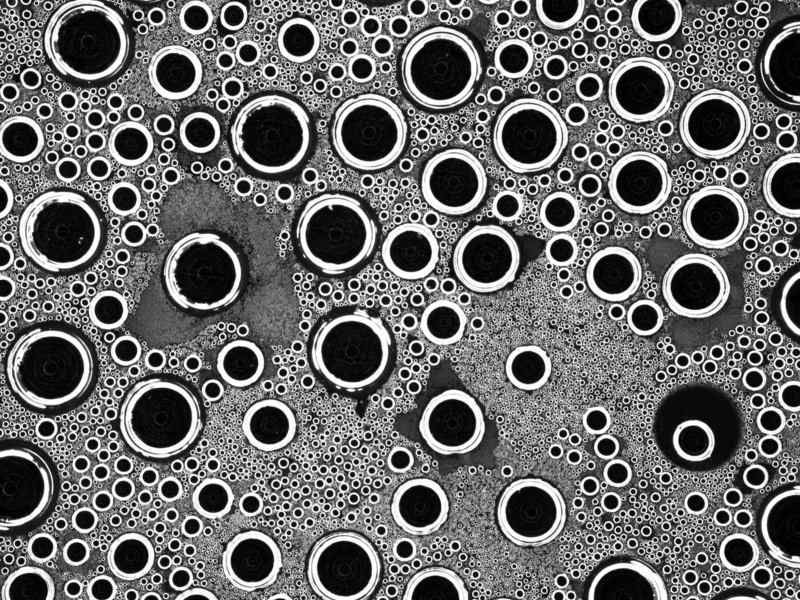
A large drop can grow by swallowing up the smaller drops at its edge.
© MPI for Dynamics and Self-Organisation

When moisture impinges upon a cooler surface, the following series of events takes place; individual water molecules first coalesce into tiny droplets, being only a few micro-metres in size. While these grow, smaller ones constantly repopulate the spaces between them, and can coalesce with their larger siblings over time. Up to now, researchers have assumed that the distribution of droplet sizes followed a power law. This states that irrespective of whether the early, middle or late growth phase is involved, the majority of droplets are small, and the larger the droplets are, the fewer of them are present. These kinds of power laws describe numerous distributions in nature and in technology, for example the size of craters on the moon, the frequency distribution of chemical elements in the earth’s crust, and of words in texts.
However, the newest results of the Göttingen-based scientists show that one and the same law does not aptly describe the entire growth phrase of the droplets. Their behaviour does not follow the precepts of scaling theory, especially at the beginning of their growth – the early childhood of the droplets, so to speak. “No doubt, there are many more small drops than large ones, just as previously predicted. Still, significantly fewer small droplets are observed than expected,” says Jürgen Vollmer, the scientist at the Max-Planck Institute for Dynamics and Self-Organisation leading the study, while describing the results. The researchers explain this by the fact that the small drops grow out of their childhood more rapidly than required by scaling theory. In addition, during the final growth phase, the droplet growth speed had to be augmented.
To detect these features, precise observation is required. Jürgen Vollmer and Björn Hof, who also conducts research at the Max Planck Institute in Göttingen, practise the art of “drop counting” with their team in two ways: experimentally and in computer simulations. “The basic setup of the experiment is reminiscent of a pot of boiling water with a lid,” says Tobias Lapp, who carried out the majority of the experiments. In the experiment, water is heated under carefully controlled conditions in a container that is sealed with transparent cover. A camera that is mounted above the cover takes a snapshot image of the droplet pattern six times per second. The images are then evaluated with a specially developed computer program. “To evaluate each image, each drop must be identified and assigned to a specific size category,” says Lapp.
The other leg of the study is numerical simulation. “How moisture condenses on a surface can be very well modelled using a computer,” explains Vollmer. In the simulations water impinges uniformly on a surface and initially forms tiny droplets that gradually grow due to the on-going precipitation of water. Frequently there are cascading collisions in which several smaller droplets merge into a larger one. “To trace the growth process exactly, we have to account for young, tiny drops just the same as for the significantly older, larger ones,” says Johannes Blaschke in explanation of the method’s difficulty. Blaschke developed and carried out the simulations as part of his master's thesis. In addition, it was necessary to average over several hundred simulations. “This sort of numerical effort had not been undertaken by anyone else in this field until now.”
The pay-off for the sophisticated droplet counting: the researchers could identify a significant kink in the early growth, as well as corrections to the growth speed in the very late phase. “The power law thus no longer holds,” summarises Vollmer, adding: “In the experiments there are more exceptions than instances that go by the old rules."
To explain the new results, droplet growth must be observed very carefully. There are fundamentally three mechanisms by which an existing droplet can increase in size: two drops which are more or less equal in size can coalesce, a small droplet can grow until its edge touches the edge of a significantly larger one and thus be swallowed up, or the droplets can grow via precipitation. In the beginning, small droplets are still widely separated and therefore collect the precipitate especially efficiently. The water that lands in their vicinity migrates over the surface toward them, which speeds up their growth. Near the end of the growth process, there are already so many drops nearby that the neighbours absorb these “indirect hits”. In contrast, the especially large drops hardly meet any others which are larger, and so there are none to swallow them up – consequentially, their growth slows.
The researchers’ results could benefit technical applications that are based on the special properties of drops. Since droplets can absorb heat effectively, they are especially needed in refrigeration and air conditioning to name a few. An improved understanding of the processes by which they develop can facilitate energy-saving methods and foster mildew-free homes. Furthermore, modern efficient irrigation methods also depend on creating droplets of specific sizes. Hence, in-depth knowledge of the features of droplet growth could be of assistance there as well.

































































