Carnegie Mellon researchers use NMR to determine whether gold nanoparticles exhibit 'handedness'
Carnegie Mellon University's Roberto R. Gil and Rongchao Jin have successfully used NMR to analyze the structure of infinitesimal gold nanoparticles, which could advance the development and use of the tiny particles in drug development.
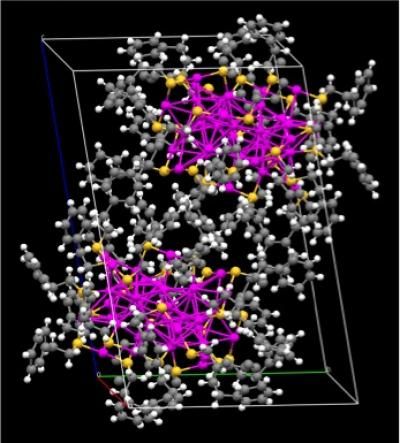
Pictured is the crystal structure of a pair of gold nanoparticles that exist in a right-handed (bottom) and left-handed (top) configuration. These nanoparticles hold great promise as a chiral catalyst -- a tool highly sought-after by the pharmaceutical industry.
Carnegie Mellon University
Their approach offers a significant advantage over routine methods for analyzing gold nanoparticles because it can determine whether the nanoparticles exist in a both right-handed and left-handed configuration, a phenomenon called chirality. Determining a nanoparticle's chirality is an important step toward developing them as chiral catalysts — tools that are highly sought-after by the pharmaceutical industry. Their results are published online at ACS Nano.
Many drugs on the market today contain at least one molecule that is chiral. Often only one of the configurations, or isomers, is effective in the body. In some cases, the other isomer may even be harmful. A striking example is the drug thalidomide, which consisted of two isomers: one of which helped pregnant women control nausea while the other caused damage to the developing fetus. In an effort to create safer, more effective drugs, drug manufacturers are looking for ways to produce purer substances that contain only the left- or right-handed isomer.
Huifeng Qian, a fourth-year graduate student working with Jin, created a gold nanoparticle that has the potential to catalyze chemical reactions that will produce one isomer rather than the other. The nanoparticle is comprised of precisely 38 gold atoms and measures a mere 1.4 nanometers. Qian worked diligently for nearly a year to grow the nanoparticles into high-quality crystals so that he could study their structure using x-ray crystallography.
"Growing a pure crystal from nanoparticles is very challenging, and you may not even be able to get a crystal at all," said Jin, an assistant professor of chemistry in CMU's Mellon College of Science. "In the nanoparticle community, the crystal structures of only three nanoparticles have been reported."
In Jin's case, x-ray crystallography revealed that the gold nanoparticle is chiral. Chemists typically probe the internal chiral structure of gold nanoparticles using a technique called circular dichoism spectroscopy. When pure chiral molecules are exposed to circularly polarized light, each isomer absorbs the light differently, resulting in a unique — and of opposite sign — spectrum for each isomer. The process of creating the gold nanoparticles, however, often results in a 50/50 mix of each isomer, known as racemates.
"Because the spectrum is of opposite sign for each isomer, they cancel each other out and the net optical response is zero. This makes circular dichoism (CD) spectroscopy useless when it comes to determining the chirality of gold nanoparticles in 50/50 mixtures," said Gil, associate research professor of chemistry and director of the Department of Chemistry's NMR Facility.
Since Jin couldn't use circular dichoism spectroscopy, Gil was able to use NMR to help Jin distinguish between his gold nanoparticles' left- and right-handed isomers.
NMR spectroscopy takes advantage of the physical phenomenon wherein some nuclei wobble and spin like tops, emitting and absorbing a radio frequency signal in a magnetic field. By observing the behavior of these spinning nuclei, scientists can piece together the chemical structure of the compound.
In 1957, scientists observed that the hydrogen atoms of a freely rotating methylene (CH2) group produced two different frequencies if they were close to a chiral center. Jin's gold nanoparticles, which have a chiral core, are cushioned by several chemical groups, including freely rotating methylene groups. Gil reasoned that the nanoparticles' chiral core should induce the methylene group's two hydrogen atoms to give off different frequencies, a phenomenon known as diastereotopicity.
Gil and Jin compared the NMR signal from the hydrogen atoms in a non-chiral gold nanoparticle with the NMR signal from the hydrogen atoms in chiral gold nanoparticle. The non-chiral nanoparticle's NMR spectrum did not reveal any differences, but the chiral nanoparticle's NMR spectrum revealed two different hydrogen signals, providing a simple and efficient way of telling whether the particle is chiral or not, even for a 50/50 mixture of isomers.
"NMR is an alternative — and very efficient — method for providing useful information about how the atoms in nanoparticles form the molecular structure. Because NMR can determine chirality in some cases, it can readily be used to determine the purity of a nanoparticle mixture," Jin said.
In current work, Jin and Qian are striving to turn their 50/50 mixture of right- and left-handed isomers into a pure solution of one or the other.
Other news from the department science
These products might interest you
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!

Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!