The hidden danger of oxygen
Long-lived reactive oxygen intermediates that develop on particles in the atmosphere could explain why more and more people are suffering from allergies
New findings from researchers at the Max Planck Institute for Chemistry and the Paul Scherrer Institute in Switzerland help to explain how toxic and allergy-causing substances in our air are formed. The scientists have for the first time detected long lived reactive oxygen intermediates on the surface of aerosol particles. These forms of oxygen survive here for more than 100 seconds and in that time react with other air pollutants such as nitrogen oxides. Chemically speaking, the dust particles are oxidized and nitrated. This is what makes soot particles more toxic and increases the potential of pollen to cause allergies.
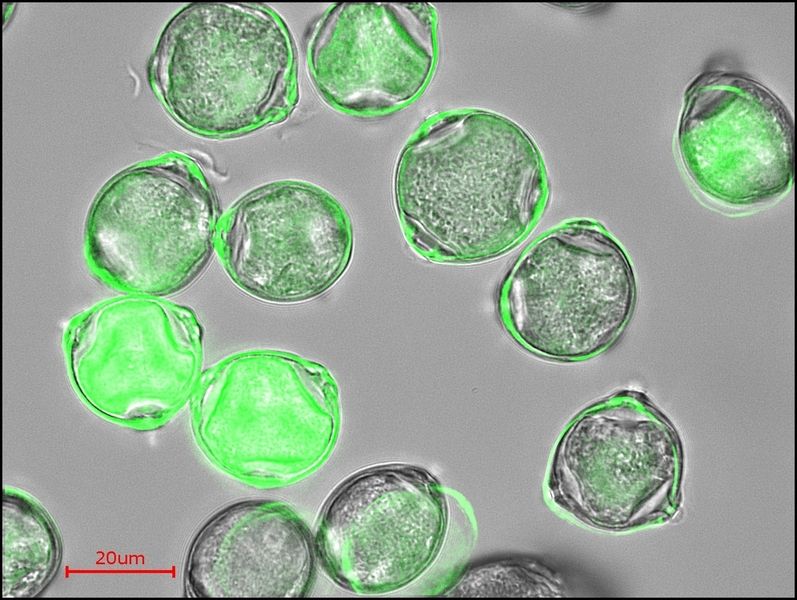
Birch pollen with allergenic potential. The colouring of the fluorescence microscopic picture shows the difference in the chemical composition of the pollen which can contain allergy-triggering protein in the cell and on its surface.
© Manabu Shiraiwa/MPI for Chemistry
Although scientists have suspected for years that these intermediate forms exist, it was believed that they disappeared within a fraction of a second, and therefore had little impact on chemical processes in the atmosphere. The intermediate forms of oxygen develop when ozone reacts with particulate matter such as soot, polycyclic aromatic hydrocarbons or pollen proteins.
"Not only does our research resolve earlier contradictions between theoretical calculations and measurements, it also shows that intermediates are also responsible for many atmospheric and physiological reactions," said Manabu Shiraiwa, lead author of the study.
Ulrich Pöschl, head of the aerosol research group at the Max Planck Institute in Mainz, goes one step further: "We suspect that the increase in allergies in industrialized countries is linked to these reactions. The more ozone and nitrogen oxides that are emitted by industry and traffic, the more frequently organic molecules such as birch pollen proteins are being nitrated and this is what irritates our immune system.” Pöschl and his colleagues have obtained evidence that these nitrated proteins can indeed cause more severe allergic reactions than the native protein form. If this hypothesis is confirmed, human health would be at even greater risk from combustion-related emissions than previously thought.
The reactive oxygen intermediates may also explain some of the direct adverse health effects of diesel soot and tobacco smoke particles. The polycyclic aromatic hydrocarbons found on the surface of these particles again readily react with ozone and form long-lived reactive oxygen intermediates. If the particles are inhaled, they interact directly with physiological processes in the human lung and other organs.
The scientists assume also that the oxygen intermediates may have an indirect effect on our climate. Presumably they are involved in the formation and growth of fine organic particles from volatile organic compounds emitted from both natural and manufactured sources such as vegetation and industrial activities. These particles scatter sunlight and influence the formation of clouds and precipitation, thus affecting the Earth’s energy balance and the hydrological cycle.
To quantify the atmospheric abundance and climatic effects of reactive oxygen intermediates, the Mainz-based Max Planck researchers will perform further kinetic experiments and extensive, numerical simulations. In collaboration with biomedical partners, they are also investigating the physiological effects of nitrated proteins formed by the oxygen intermediates reacting with nitrogen oxides.
Original publication
Manabu Shiraiwa, Yulia Sosedova, Aurélie Rouvière, Hong Yang, Yingyi Zhang, Jonathan P. D. Abbatt, Markus Ammann and Ulrich Pöschl; "The role of long-lived reactive oxygen intermediates in the reaction of ozone with aerosol particles"; Nature Chemistry, 20. Februar 2011
Original publication
Manabu Shiraiwa, Yulia Sosedova, Aurélie Rouvière, Hong Yang, Yingyi Zhang, Jonathan P. D. Abbatt, Markus Ammann and Ulrich Pöschl; "The role of long-lived reactive oxygen intermediates in the reaction of ozone with aerosol particles"; Nature Chemistry, 20. Februar 2011
Topics
Organizations
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Aviation_fuel
Uhdenora receives order from AO "KAUSTIK" Kazakhstan

Labomatic and KNAUER enter into strategic cooperation - Customers in the field of liquid chromatography in particular are likely to benefit from the complementary expertise
Emerson Enters into Supplier Services Agreement with Fluor Corporation to Provide Automation and Control Solutions and Services
Robert_W._Bussard
Robert_J._LeRoy