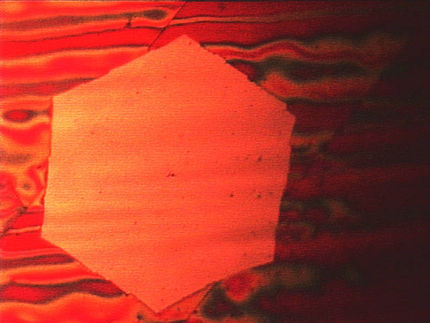
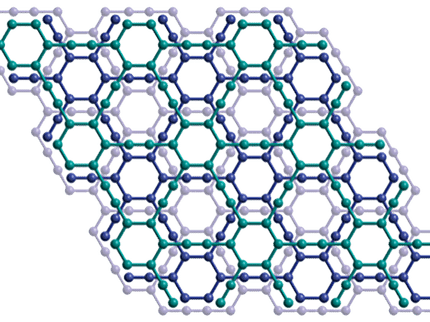
Sugar and slice make graphene real nice
Rice University lab finds table sugar, metallic sheets produce pristine graphene in one step
Future computers may run a little sweeter, thanks to a refinement in the manufacture of graphene at rice University.
Rice researchers have learned to make pristine sheets of graphene, the one-atom-thick form of carbon, from plain table sugar and other carbon-based substances. They do so in a one-step process at temperatures low enough to make graphene easy to manufacture.
The lab of Rice chemist James Tour reported in the online version of the journal Nature that large-area, high-quality graphene can be grown from a number of carbon sources at temperatures as low as 800 degrees Celsius (1,472 F). As hot as that may seem, the difference between running a furnace at 800 and 1,000 degrees Celsius is significant, Tour said.
"At 800 degrees, the underlying silicon remains active for electronics, whereas at 1,000 degrees, it loses its critical dopants," said Tour, Rice's T.T. and W.F. Chao Chair in Chemistry as well as a professor of mechanical engineering and materials science and of computer science.
Zhengzong Sun, a fourth-year graduate student in Tour's lab and primary author of the paper, found that depositing carbon-rich sources on copper and nickel substrates produced graphene in any form he desired: single-, bi- or multilayer sheets that could be highly useful in a number of applications.
Sun and his colleagues also found the process adapts easily to producing doped graphene; this allows the manipulation of the material's electronic and optical properties, which is important for making switching and logic devices.
For pristine graphene, Sun started with a thin film of poly (methyl methacrylate) (PMMA) -- perhaps best known in its commercial guise as Plexiglas -- spun onto a copper substrate that acted as a catalyst. Under heat and low pressure, flowing hydrogen and argon gas over the PMMA for 10 minutes reduced it to pure carbon and turned the film into a single layer of graphene. Changing the gas-flow rate allowed him to control the thickness of the PMMA-derived graphene.
Then it got more interesting, Sun said. He turned to other carbon sources, including a fine powder of sucrose -- aka table sugar. "We thought it would be interesting to try this stuff," Sun said. "While other labs were changing the metal catalysts, we tried changing the carbon sources."
Sun put 10 milligrams of sugar (and later fluorene) on a square-centimeter sheet of copper foil and subjected it to the same reactor conditions as the PMMA. It was quickly transformed into single-layer graphene. Sun had expected defects in the final product, given the chemical properties of both substances (a high concentration of oxygen in sucrose, five-atom rings in fluorene); but he found potential topological defects would self-heal as the graphene formed.
"As we looked deeper and deeper into the process, we found it was not only interesting, but useful," Sun said.
He tried and failed to grow graphene on silicon and silicon oxide, which raised the possibility of growing patterned graphene from a thin film of shaped copper or nickel deposited onto silicon wafers.
Doped graphene opens more possibilities for electronics use, Tour said, and Sun found it fairly simple to make. Starting with PMMA mixed with a doping reagent, melamine, he discovered that flowing the gas under atmospheric pressure produced nitrogen-doped graphene. Pristine graphene has no bandgap, but doped graphene allows control of the electrical structure, which the team proved by building field-effect transistors.
"Each day, the growth of graphene on silicon is approaching industrial-level readiness, and this work takes it an important step further," Tour said.
Topics
Organizations
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Metal
Category:Meglitinides

Bubble-recoil could be used to cool microchips, even in space
Electron pairs precede high-temperature superconductivity - New method exploring 'energy gap' shows electron pairs exist before superconductivity sets in
Manipulation of the characteristics of magnetic materials
Inorganic materials display massive and instantaneous swelling and shrinkage
Lipoic_acid
TSKgel HT2 Series Columns | GPC columns | Tosoh
Vesuvianite
Category:EC_3.11
Table_of_nuclides,_73-96