Dionex Demonstrates Simplified Determination of 2-Ethylhexanoic Acid in Clavulanate
Dionex announced a simplified method for the detection and quantification of 2-ethylhexanoic acid, a potential impurity in potassium clavulanate active pharmaceutical ingredient (API) incorporated during sample purification. Application Note 262: Determination of 2-Ethylhexanoic Acid Impurity in Clavulanate reports results for limit of detection/limit of quantification (LOQ/LOQ), linear calibration range, spike recovery, retention time precision, and peak area precision determinations that show this to be an accurate and reproducible technique to determine 2-ethylhexanoic acid in clavulanate below the 0.8% acceptance criteria.
The determination method outlined in Application Note 262 uses 150-fold lower concentration of the API and eliminates the need for solvent extraction per the current United States Pharmacopeia (USP) monograph, thus consuming less of the API and simplifying sample preparation.
Most read news
Other news from the department research and development
These products might interest you

AZURA Purifier + LH 2.1 by KNAUER
Preparative Liquid Chromatography - New platform for more throughput
Save time and improve reproducibility during purification
Pharmaceutical Substances by Thieme Verlag
Look up Industrial Syntheses of 2,600 APIs
Your tool for Syntheses, Patents and Applications – Pharmaceutical Substances

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Lenzing and Acordis - US Joint Venture
Mitsui Chemicals to License PP Process to Thai Polypropylene
Tripos Completes Acquisition of Optive Research, a Molecular Discovery Software Company
Accelrys Announces Pfizer Inc. as the First Member of the Enterprise Cheminformatics Consortium - Consortium to Drive the Evolution of Enterprise Cheminformatics Systems Using Open and Flexible Service-Oriented Architecture
The buzz on an amazing new mosquito repellent: Will it fly?
Orion sues generic drug companies in the U.S. to enforce patents for entacapone
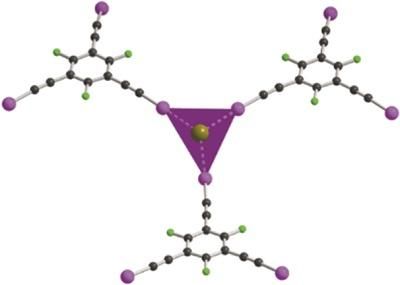
Coordination chemistry of anions through halogen-bonding interactions
Katz_Group_of_Companies
GEA: Dr. Stephan Petri to continue as Member of the Executive Board until 2018

Rapid Change: Ceresana Analyzes the Global Market for Printing Inks




























































