Turning over a new leaf
Researchers have transformed the skeleton of a leaf into iron carbide. The new technique enables the conversion of metal carbides into intricate microstructures in just one step.
As recently confirmed by a group of chemists from the Max Planck Institute of Colloids and Interfaces, nature can provide very useful templates for technical applications. The scientists have devised a new process involving the almost complete conversion of a leaf skeleton into magnetic iron carbide. To do this, they treated the leaf with iron acetate, nitrogen and heat. This technique can be used to recreate all natural carbonaceous structures with metal carbides. The result is not just beautiful, but also very useful. Biology’s intricate forms provide a wide range of templates for a variety of applications.
Nature’s fine structures are also suitable for technical applications - they exist in a myriad variety of forms, they usually display high mechanical stability and, due to their large surfaces, provide suitable templates for catalysts and electrodes. Researchers from the Max Planck Institute of Colloids and Interfaces in Potsdam have now succeeded in converting the filigree skeleton of a leaf into iron carbide using a very simple method. Materials scientists are interested in metal carbides because they are magnetic, conduct electricity and can withstand both high temperatures and mechanical stress. However, due to the stability of the material, it has proven almost impossible thus far to convert it into a specific form.
The Potsdam-based chemists have now succeeded in doing this in a very simple way. They started by dipping the leaf skeleton of leaves from a rubber tree into an iron acetate solution. They then air-dried the soaked skeleton at 40 degrees Celsius before treating it with nitrogen gas and heating it to 700 degrees Celsius. "The structure was conserved down to the last detail," says Zoe Schnepp, who carried out the experiment.
When heated, the iron acetate in the leaf skeleton is converted into iron oxide, which is then reduced by the carbon in the leaf skeleton to iron carbide. "The skeleton provides both the basis for the form and the carbon for the reaction," says Zoe Schnepp. "As a result, we can convert the organic substance in just one step. This is what distinguishes our method from other techniques which also use biological forms as templates for inorganic structures." Researchers have been producing metal oxides on the basis of natural materials like leaves for some time now. "One team has already succeeded in generating silicon carbide from pre-treated natural materials," says Zoe Schnepp. "We’ve now developed this process even further."
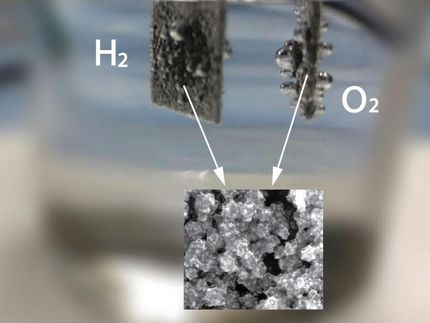
To test whether the leaf was fully converted into iron carbide, the researchers hung it in an electrolytic cell as an anode. Oxygen from the cell bubbled at the leaf and hydrogen bubbles rose at the cathode. "The experiment confirms that most of the leaf was converted into iron carbide. Apart from that, it only contains a bit of carbon," says Zoe Schnepp. The researchers also used a permanent magnet to demonstrate that the leaf had acquired the magnetic characteristics of the iron carbide.
The new method should function with all natural carbonaceous materials. "We would now like to test it on other materials," says Schnepp. "What is important about this study is that it shows how we can exploit nature’s formal variety to produce wafer-thin metal carbide structures in one simple step."
Original publication: Zoe Schnepp, Wen Yang, Markus Antonietti, Cristina Giordano Biotemplating of Metal Carbide Microstructures; "The Magnetic Leaf"; Angewandte Chemie, International Edition, August 16, 2010
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Fatty_acid
Ministry_of_Health,_Labour_and_Welfare_(Japan)
Management change in Biesterfeld
Louis_Nicolas_Vauquelin

BASF inaugurates enzyme-based production plant for biocatalyzed acrylamide
Higher_sulfur_oxides
Topicity
Vladimir_Galkin
Category:Neptunium
Wittenoom,_Western_Australia