Ultrafast dissociation of molecules studied at BESSY II
Findings could deepen our understanding of chemical reactions at the molecular level
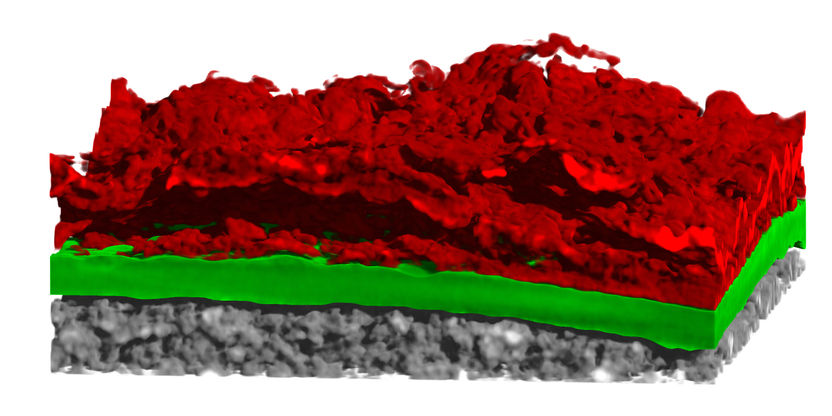
For the first time, an international team has tracked at BESSY II how heavy molecules – in this case bromochloromethane – disintegrate into smaller fragments when they absorb X-ray light. Using a newly developed analytical method, they were able to visualise the ultrafast dynamics of this process. In this process, the X-ray photons trigger a "molecular catapult effect": light atomic groups are ejected first, similar to projectiles fired from a catapult, while the heavier atoms - bromine and chlorine - separate more slowly.

The X-ray photons trigger a ‘molecular catapult effect’: light atomic groups are ejected first, similar to projectiles shot from a catapult, while the heavier atoms – bromine and chlorine – separate much more slowly. The image was printed on the cover of "The Journal of Physical Chemistry Letters".
© The Journal of Physical Chemistry Letters
When X-rays hit molecules, they can knock electrons out of certain orbitals and into extremely high-energy states, breaking chemical bonds. This often happens ultra rapidly, in just a few femtoseconds (10-15 s). While this phenomenon has been studied in light molecules such as ammonia, oxygen, hydrochloric acid or simple carbon compounds, it has hardly been studied in molecules with heavier atoms.
A team from France and Germany has now studied the rapid decay of molecules containing halogens. They focused on a molecule in which bromine and chlorine atoms are linked by a light bridge - an alkylene group (CH2). The measurements were made at the XUV beamline of BESSY II.
The absorption of the X-rays caused molecular bonds to break, creating ionic fragments that could be analysed. The scientists were able to produce a visualisation from the measurement data. It shows how the atoms move in the fleeting intermediate states just before the bonds break. To do this, the team developed a new method of analysis called IPA (Ion Pair Average) and combined it with ab initio theoretical calculations to reconstruct the processes.
The results show that light groups of atoms such as CH2 are ejected first, while the heavier atoms - bromine and chlorine - are left behind and therefore separate more slowly. Interestingly, this catapult-like behaviour only occurs at certain X-ray energies. Theoretical simulations, in agreement with experimental observations, emphasise the crucial role of vibrations of the lighter groups of atoms in triggering these ultrafast reactions.
“This study highlights the unique dynamics of molecular dissociation upon X-ray irradiation," says Dr Oksana Travnikova (CNRS, Université Sorbonne, France), first author of the study now published in J. Phys. Chem. Lett. In particular, it shows that the catapult-like motion of light groups initiates the separation of heavy fragments, a process that unfolds in a remarkably short time. These findings could deepen our understanding of chemical reactions at the molecular level and how high-energy radiation affects complex molecules.
Original publication
Oksana Travnikova, Victor Kimberg, Barbara Cunha de Miranda, Florian Trinter, Markus S. Schöffler, Stéphane Carniato, Tatiana Marchenko, Renaud Guillemin, Iyas Ismail, Gregor Kastirke, Maria Novella Piancastelli, Till Jahnke, Reinhard Dörner, Marc Simon; "X-ray-Induced Molecular Catapult: Ultrafast Dynamics Driven by Lightweight Linkages"; The Journal of Physical Chemistry Letters, Volume 15, 2024-11-21
Original publication
Oksana Travnikova, Victor Kimberg, Barbara Cunha de Miranda, Florian Trinter, Markus S. Schöffler, Stéphane Carniato, Tatiana Marchenko, Renaud Guillemin, Iyas Ismail, Gregor Kastirke, Maria Novella Piancastelli, Till Jahnke, Reinhard Dörner, Marc Simon; "X-ray-Induced Molecular Catapult: Ultrafast Dynamics Driven by Lightweight Linkages"; The Journal of Physical Chemistry Letters, Volume 15, 2024-11-21
Organizations
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.