Nearly 200-year-old challenge in polymers solved in major materials breakthrough
Researchers defy materials science rules with molecules that release stored length to decouple stiffness and stretchability
Advertisement
Researchers at the University of Virginia School of Engineering and Applied Science have developed a new polymer design that appears to rewrite the textbook on polymer engineering. No longer is it dogma that the stiffer a polymeric material is, the less stretchable it has to be.

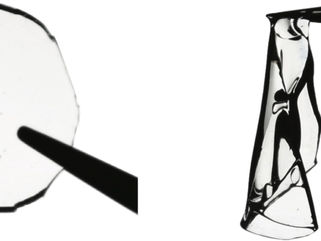
An artistic rendering of a network formed by crosslinking foldable bottlebrush polymers, which feature a collapsed backbone grafted with many flexible linear side chains.
Liheng Cai, Baiqiang Huang/Soft Biomatter Lab, University of Virginia School of Engineering and Applied Science

Baiqiang Huang (left), a Ph.D. student in the Department of Materials Science and Engineering at the University of Virginia, with UVA assistant professor Liheng Cai.
Matt Cosner, University of Virginia School of Engineering and Applied Science


“We are addressing a fundamental challenge that has been thought to be impossible to solve since the invention of vulcanized rubber in 1839,” said Liheng Cai, an assistant professor of materials science and engineering, and chemical engineering.
That’s when Charles Goodyear accidentally discovered that heating natural rubber with sulfur created chemical crosslinks between the strand-like rubber molecules. This crosslinking process creates a polymer network, transforming the sticky rubber, which melts and flows in the heat, into a durable, elastic material.
Ever since, it’s been believed that if you want to make a polymer network material stiff, you have to sacrifice some stretchability.
That is, until Cai’s team, led by Ph.D. student Baiqiang Huang, proved otherwise with their new “foldable bottlebrush polymer networks.” Their work, funded by Cai’s National Science Foundation CAREER Award, is on the cover of the Nov. 27 issue of Science Advances.
‘Decoupling’ Stiffness and Stretchiness
“This limitation has held back the development of materials that need to be both stretchable and stiff, forcing engineers to choose one property at the expense of the other,” Huang said. “Imagine, for example, a heart implant that bends and flexes with each heartbeat but still lasts for years.”
Huang first-authored the paper with postdoctoral researcher Shifeng Nian and Cai.
Crosslinked polymers are everywhere in products we use, from automobile tires to home appliances — and they are increasingly used in biomaterials and health care devices.
Some applications the team envisions for their material include prosthetics and medical implants, improved wearable electronics, and “muscles” for soft robotic systems that need to flex, bend and stretch repeatedly.
Stiffness and extensibility — how far a material can stretch or expand without breaking — are linked because they originate from the same building block: the polymer strands connected by crosslinks. Traditionally, the way to stiffen a polymer network is to add more crosslinks.
This stiffens the material but doesn’t solve the stiffness-stretchability trade-off. Polymer networks with more crosslinks are stiffer, but they don’t have the same freedom to deform, and they break easily when stretched.
“Our team realized that by designing foldable bottlebrush polymers that could store extra length within their own structure, we could ‘decouple’ stiffness and extensibility — in other words, build in stretchability without sacrificing stiffness,” Cai said. “Our approach is different because it focuses on the molecular design of the network strands rather than crosslinks.”
How the Foldable Design Works
Instead of linear polymer strands, Cai’s structure resembles a bottlebrush — many flexible side chains radiating out from a central backbone.
Critically, the backbone can collapse and expand like an accordion that unfolds as it stretches. When the material is pulled, hidden length inside the polymer uncoils, allowing it to elongate up to 40 times more than standard polymers without weakening.
Meanwhile, the side chains determine stiffness, meaning that stiffness and stretchability can finally be controlled independently.
This is a “universal” strategy for polymer networks because the components that make up the foldable bottlebrush polymer structure are not restricted to specific chemical types.
For example, one of their designs uses a polymer for the side chains that stays flexible even in cold temperatures. But using a different synthetic polymer, one that is commonly used in biomaterial engineering, for the side chains can produce a gel that can mimic living tissue.
Like many of the novel materials developed in Cai’s lab, the foldable bottlebrush polymer is designed to be 3D-printable. This is true even when mixed with inorganic nanoparticles, which can be designed to exhibit intricate electric, magnetic or optical properties.
For example, they can add conductive nanoparticles, such as silver or gold nanorods, which are critical to stretchable and wearable electronics.
“These components give us endless options for designing materials that balance strength and stretchability while harnessing the properties of inorganic nanoparticles based on specific requirements,” Cai said.