Sustainably producible covalent organic networks capture carbon dioxide
"For the first time, a solid-phase synthesis of COFs has been developed that works completely without solvents"
An international research team led by Heinrich Heine University Düsseldorf (HHU) and the University of Siegen has synthesized a new compound that forms a so-called covalent organic network. The phosphonic acid-based compound is stable and can be used, for example, to store carbon dioxide (CO2), as the researchers describe in the journal Nature Communications.
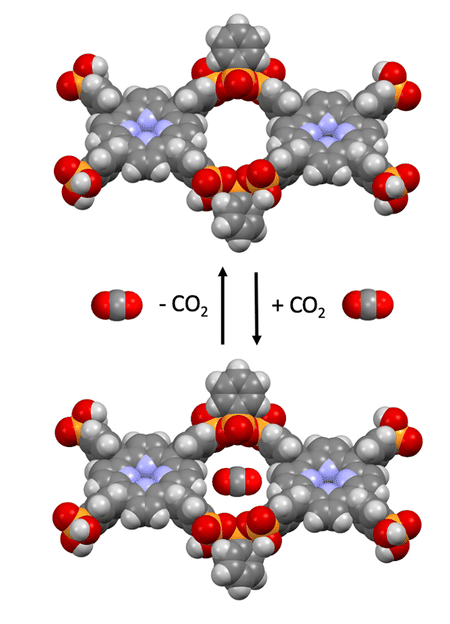
Based on phosphonic acid, the research team has developed a stable covalent organic network that is able to store carbon dioxide (CO2).
HHU / Gündog Yücesan
Covalent organic frameworks (COFs) are a class of porous crystalline materials that form scaffold structures. The term "covalent" means that chemical bonds are formed between individual building blocks of the network through shared electron pairs.
A research team led by Dr. Gündoğ Yücesan, Heisenberg Junior Research Group Leader at the Chair of Nanoporous and Nanoscale Materials at HHU and Prof. Dr. Jörn Schmedt auf der Günne, Head of the Institute of Inorganic Materials Chemistry at the University of Siegen, has now presented a simple approach to this family of networks, whose members are particularly stable and which promise high application potential. Researchers from Berlin, Bremen, Saarbrücken, Turkey and the United Kingdom were also involved in the study published in Nature Communications.
The class of polyphosphonate scaffold compounds is characterized by phosphorus-oxygen-phosphorus bonds, which consist of building blocks of simple organic phosphonic acids and can be assembled by heating them to temperatures of only around 200 degrees Celsius.
Dr. Yücesan: "These COFs have the special property that they are stable even in the presence of water and water vapour despite the mild synthesis conditions and can therefore be used in water and electrolytes, unlike previously developed compounds."
Another milestone was the development of a sustainable synthesis process. Yücesan: "For the first time, a solid-phase synthesis of COFs has been developed that works completely without solvents. This method enables cost-effective and scalable production on a kilogram to ton scale, which makes it more economical compared to other microporous materials."
One challenge for the researchers was that the compounds did not crystallize well or were amorphous. They were able to demonstrate the cross-linking using the nuclear magnetic resonance method. Prof. Dr. Schmedt auf der Günne explains: "If we had not been able to use the common states of neighboring phosphorus nuclei, the cross-linking structure of the substance would have remained in the dark and its properties would not have been understood."
Polyphosphonates of this type have a high application potential. The climate-damaging greenhouse gasCO2 is bound by their networks. It can be released again by a slight change in pressure. "Such substances are needed for exhaust gas purification and to avoid greenhouse gas emissions," the authors of the study state.
Note: This article has been translated using a computer system without human intervention. LUMITOS offers these automatic translations to present a wider range of current news. Since this article has been translated with automatic translation, it is possible that it contains errors in vocabulary, syntax or grammar. The original article in German can be found here.
Original publication
Ke Xu, Robert Oestreich, Takin Haj Hassani Sohi, Mailis Lounasvuori, Jean G. A. Ruthes, Yunus Zorlu, Julia Michalski, Philipp Seiffert, Till Strothmann, Patrik Tholen, A. Ozgur Yazaydin, Markus Suta, Volker Presser, Tristan Petit, Christoph Janiak, Jens Beckmann, Jörn Schmedt auf der Günne, Gündoğ Yücesan; "Polyphosphonate covalent organic frameworks"; Nature Communications, Volume 15, 2024-9-9
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.






























































