Alcohol as a storage medium
Power-to-methanol could become a mainstay of the energy transition
Advertisement
Efficient storage technologies are a key pillar of a renewable energy system for temporarily storing surplus electricity. Methanol could play an important role in this context. The big question here is how such power-to-methanol systems can be integrated into a future renewables infrastructure and operated economically. Dr. Stefan Fogel from the Helmholtz-Zentrum Dresden-Rossendorf (HZDR) has found an answer to this question through elaborate modeling and extensive simulations during his dissertation.
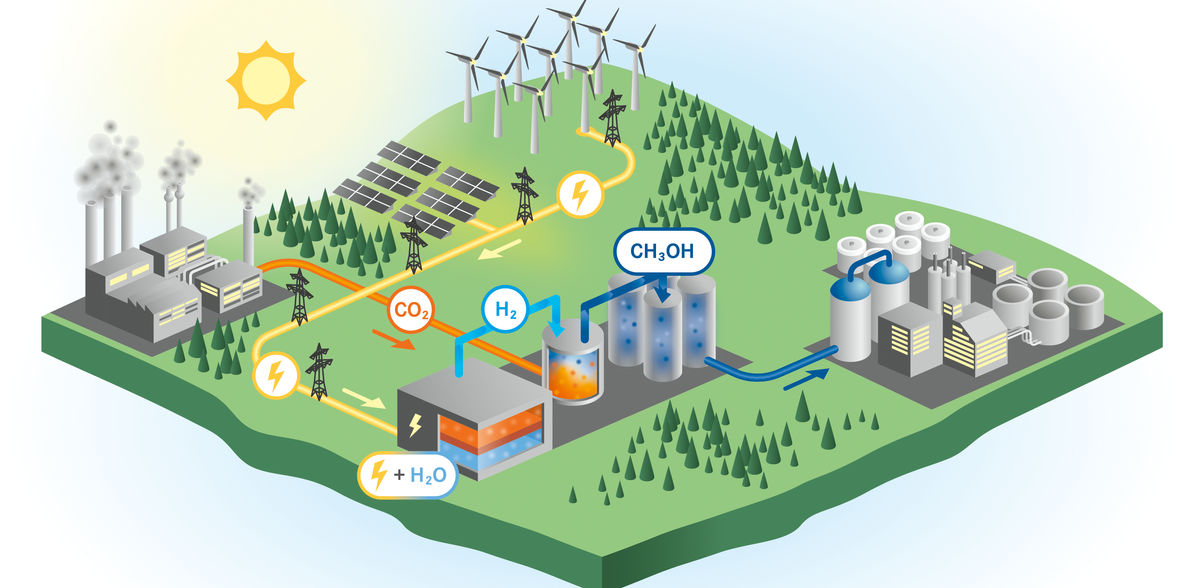
The erratic availability of sun and wind poses major challenges for a future energy system based on renewable sources. When weather conditions are ideal, more electricity is sometimes generated than the grid can take. Clever storage solutions are needed so that the systems do not have to be throttled back. One of these could be power-to-methanol. With this, surplus electricity from solar plants or wind farms is first converted into hydrogen and then, together with carbon dioxide emissions from industrial processes, into the simplest representative of alcohols.
"Methanol is a very good energy storage medium and has a much higher energy density by volume compared to hydrogen," says Dr. Stefan Fogel from the HZDR Institute of Fluid Dynamics. "As a liquid, it is also much easier to transport and store." On the one hand, this makes alcohol an ideal storage medium. On the other hand, it is also an important raw material in the chemical industry. However, how the production process can be integrated into a regenerative energy system has not yet been fully researched.
"Work on the stationary and dynamic modeling and simulation of power-to-methanol processes based on high-temperature electrolysers has so far been severely underrepresented in the scientific literature," explains the chemical engineer. "The same applies to their economic evaluation." Fogel has therefore focused his work on electrolysis systems that produce pure hydrogen at operating temperatures above 600 degrees Celsius. This is used directly in the synthesis stage without any further separation effort. This is more efficient than today's established technologies such as alkaline electrolysis.
Digital twin shows potential
He used the digital twin that he modeled of the power-to-methanol system for extensive simulations. "I looked at what happens when you operate the system dynamically," he explains in detail. The question is particularly essential with regard to renewable energies. This is because today's electrolysers are usually designed for round-the-clock operation. However, when connected decentrally to a wind farm or photovoltaic system, the systems would only operate during times of energy surplus. This poses technical challenges, but also has a significant impact on the cost of the methanol produced. "It has been shown that such a process can certainly be made more flexible," explains Fogel. "In future, it would therefore be possible to couple a power-to-methanol plant with a photovoltaic or wind power plant, operate at partial load and still achieve competitive production costs."
However, his techno-economic assessment of the data obtained from the simulations shows that we are still a long way from this point today. This showed that the costs for methanol are currently not competitive, regardless of the process nesting and the technologies used. This is primarily due to the fact that fossil raw materials are still unrivaled in terms of cost due to the infrastructure that has been built up over decades. "Electrolysis technology incurs massive capital costs for investments in these plants," says Fogel. "Up to 70 percent of the costs are accounted for by the investment. In the end, the actual production costs are not that high."
However, the researcher sees this as only a temporary phase that every new technology has to go through. After all, if the market for power-to-methanol takes off in the coming years, economies of scale will reduce costs. "In my work, I also investigated how the topic could develop over the next 20 years," emphasizes the chemical engineer. To this end, he carried out extensive literature research and projected the costs into the future. The result: there will be a drastic reduction in costs. "By 2050, we could have reached the point where the power-to-methanol process is on a par with fossil fuels."
Note: This article has been translated using a computer system without human intervention. LUMITOS offers these automatic translations to present a wider range of current news. Since this article has been translated with automatic translation, it is possible that it contains errors in vocabulary, syntax or grammar. The original article in German can be found here.