Researchers achieve dual-functional supramolecular materials
Advertisement
Versatile molecular frameworks called discrete supramolecular structures act like microscopic building blocks customizable for a wide variety of applications. The structures can serve in drug delivery, provide unique environments for catalytic reactions or plug into a molecular machine.

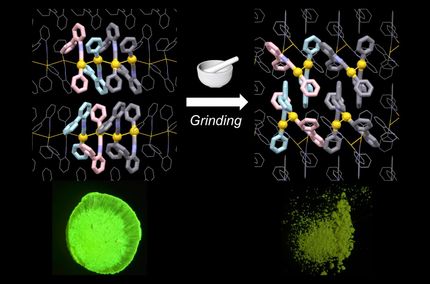
The illustration depicts alcohol and water molecules being adsorbed only into one of the two types of pores.
Yokohama National University
In their paper published June 25 in the Journal of the American Chemical Society, researchers from Yokohama National University presented a new methodology to advance self-assembly of dual-functional supramolecular materials.
Self-assembly involves the spontaneous generation of a well-defined, discrete supramolecular architecture from a given set of components under thermodynamic equilibration. Typically, a binary combination of precursors, each bearing complementary functional groups, is assembled into a stable product. Multicomponent systems, which include at least two precursors having identical functional groups, remain relatively unexplored.
Scientists are investigating methods to shepherd different precursors with the same functional groups into a unified supramolecular structure through "social self-sorting." In social self-sorting, the transitions between complex self-sorted systems mimic the regulatory function found in nature, which are capable of selective but adaptive recognition behavior.
To achieve this goal, researchers have been developing strategic approaches to prevent random incorporation and "narcissistic self-sorting," where each type of precursor assembles into independent structures.
One approach is through a technique called chiral self-sorting, which relies on the complementarity of chirality (right or left-handedness). When a racemic precursor (mixture of two chiral molecules called enantiomers) is used, both enantiomers are frequently incorporated into a single structure.
Right-handed and left-handed molecules tend to align alternately when they crystallize, and it is possible to arrange "quasi-racemates" that have slight structural differences between the right-handed and left-handed forms.
“Previous research has focused mainly on achieving the alignment of these molecules, and applying this phenomenon to the development of functional materials has been a challenge,” said corresponding author Suguru Ito, associate professor of engineering at Yokohama National University.
In their study, Ito’s team explored arranging "quasi-racemates" with slight structural differences between right-handed and left-handed forms to create crystalline materials with pores of varying sizes. The social self-sorting of two pairs of quasi-racemates was achieved by forming a ring-shaped molecule with four connecting molecules. This stable ring is obtained through a reversible reaction between the aldehyde groups of the quasi-racemates and the amine groups of the connecting molecules. As a result, the ring-shaped molecules can crystallize into porous molecular crystals featuring two types of tube-like pores.
“This represents a milestone achievement in applying the arrangement techniques of right-handed and left-handed molecules to the creation of functional materials,” Ito said.
Designing porous materials with dual-pore systems presents a complex task, yet such materials are highly valuable due to their advanced functionalities. Because each pore can be functionalized distinctly, dual-pore materials enable simultaneous multiple functions or specific designs for complex applications.
Experimental evidence confirmed that these dual pores exhibit different adsorption properties.
This study emphasizes the utility of quasi-racemates in constructing socially self-sorted supramolecular structures with two distinct functionalities. Furthermore, the methodology sets the stage for the generation of a novel class of dual-pore molecular crystals.
“To the best of our knowledge, this is the first dual-pore molecular crystal formed by socially self-sorted macrocycles,” Ito said.
Future investigations will aim to develop various multi-functional crystalline materials by applying the technique of arranging quasi-racemates.
“My ultimate goal is to establish a method for precisely arranging organic molecules and to develop functional crystalline materials that are beneficial to society,” Ito said.