Aromatic compounds: A ring made up solely of metal atoms
chemists characterise new basic structure in the field of aromaticity
Advertisement
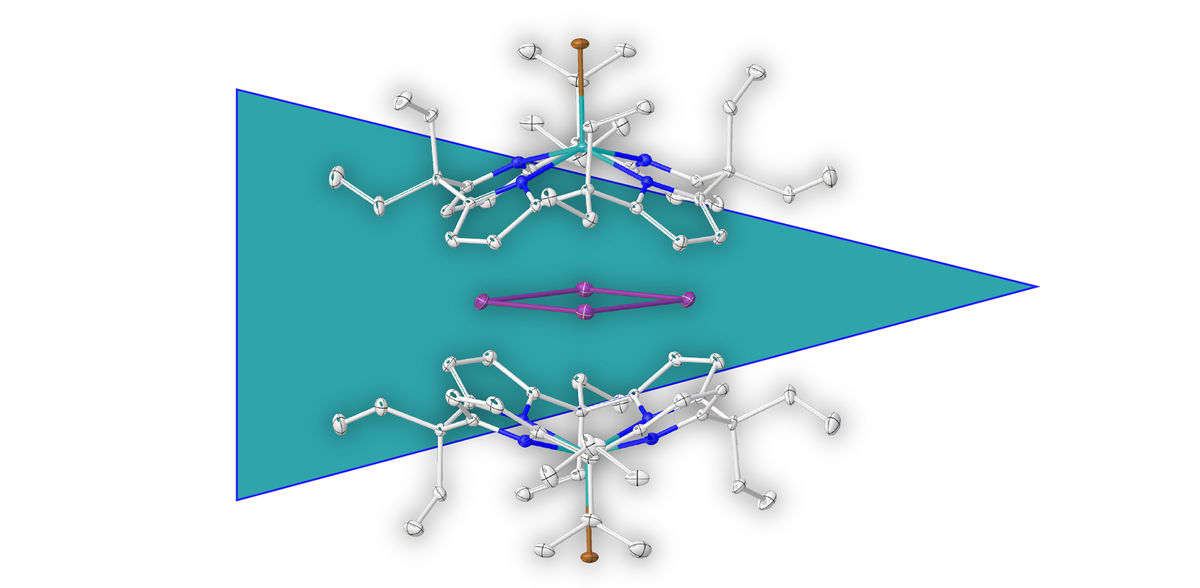
The term aromaticity is a basic, long-standing concept in chemistry that is well established for ring-shaped carbon compounds. Aromatic rings consisting solely of metal atoms were, however, heretofore unknown. The research team led by Prof. Dr Lutz Greb, a scientist at Heidelberg University’s Institute of Inorganic Chemistry, recently succeeded in isolating such a metal ring and describing it in full.
Aromatic compounds, or aromatics, are a substance class in organic chemistry, named after the aromatic smell by which the initially discovered compounds of this class were identified. In addition to ring-shaped carbon compounds, aromatic metal complexes are also known, whereby the metal atom is bound to an aromatic organic molecule. The metal ring described by the Heidelberg University chemists is different. It is made up solely of metal atoms of elemental bismuth. The isolation and characterisation of this metal ring was made possible by a new approach of supramolecular stabilisation. Lutz Greb’s research group arranged a negatively charged molecular shell around the positively charged metal ring that inhibits possible decomposition reactions.
“We assume that our approach can be used as a general method in other areas of stabilisation of positively charged rings and cages. First of all, aromatic compounds consisting solely of metal atoms aid our fundamental understanding. However, several unexpected effects of our work point to a new basic concept in the field of aromaticity. It could be significant for charge transport in metals,” stresses Prof. Greb, who heads up the research group in molecular main group chemistry at the Institute of Inorganic Chemistry.
Original publication
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.