Significant Breakthrough in Understanding of the Two-State Reactivity Mechanism
Scientists discovered first experimental methodology for measuring low-energy, spin-forbidden transitions in molecular catalysts
Iron-based catalysts are essential in various fields, including medicine, energy, and environmental science. They facilitate reactions that are vital for producing pharmaceuticals, clean fuels, and breaking down pollutants. These catalysts operate by enabling the directed transformation of molecules into desired products, and can do so using energy efficient, environmentally friendly methods. The chemistry that these iron-based catalysts are capable of depends on the oxidation- and spin-state of the iron, and changes to either of these can result in very different reactivity and products. A continuing challenge in catalysis is harnessing these differing states to increase reactivity, or the rate at which the transformation takes place, while maintaining specificity, in order to generate a majority of the desired products.
Specifically, iron(IV)-oxo complex are highly reactive intermediates involved in various oxidation reactions, such as the conversion of methane to methanol. The iron(IV)-oxo catalysts are known to have either S = 2 or S = 1 spin-states, with the S = 2 spin-states having been shown to generally be more reactive. A long-standing theory known as the two-state reactivity model suggests that the catalysts with an S = 1 ground state can switch between different spin states to drive reactions. A component of this theory is that the energy separation between these two spin-states, should be correlated with oxidative reactivity. A large challenge in probing this mechanism has been that the energy difference between these spin states had never been measured experimentally. The difficulty of measuring this excitation energy is two-fold: the transitions between these states are spin-forbidden leading to low intensity, and they are expected to be low-energy placing them outside the range of standard spectroscopic techniques.
Researchers at the Max Planck Institute for Chemical Energy Conversion and MPI für Kohlenforschung have made a significant breakthrough in understanding of the two-state reactivity mechanism. Using a combination of advanced spectroscopic techniques: resonant inelastic X-ray scattering (collected at the PEAXIS beamline at the BESSY II Helmholtz-Zentrum Berlin für Materialien und Energie) and magnetic circular dichroism, the researchers were able to directly measure the elusive triplet-quintet excitation energy in these complexes.
The implications of this research are significant: With experimental measurements of this crucial excitation, scientists can now refine their theoretical models allowing for the design of more efficient and selective catalysts. This work also demonstrates, to their knowledge, the first experimental methodology for measuring low-energy, spin-forbidden transitions in molecular catalysts.
Original publication
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Solar_chimney
Breakthrough_bleeding

Patent filings in 3D printing grew eight times faster than average of all technologies in last decade
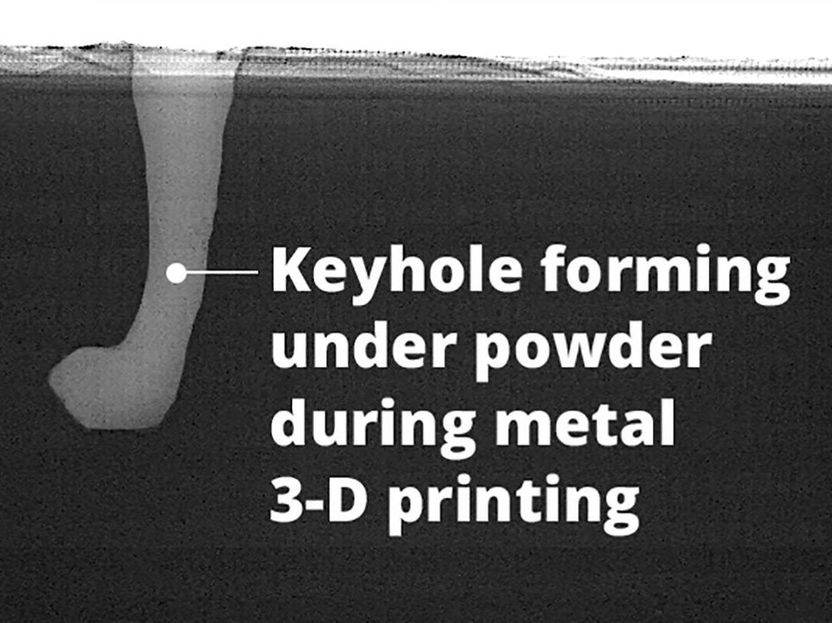
Causes for defects in 3D printing - Paving way for better results
Unique material could unlock new functionality in semiconductors - New ferroelectric material can be manipulated using light in previously impossible ways
Diphosphines

Micromeritics Ltd - Hexton, United Kingdom
Rubber_Elasticity