Chemists mimic natural molecular structures: New possibility for vaccine development
Fluorinated sugar molecules as vaccine leads against meningitis B and C
Advertisement
Meningococci (Neisseria meningitidis) are disease-causing bacteria that cause life-threatening meningitis, or blood poisoning (septicaemia). There are a number of meningococcal variants worldwide, known as serogroups, and in Germany the Standing Committee on Vaccination (STIKO) recommends immunisation against serogroups B and C in children. A team from the University of Münster (led by Prof. Dr Ryan Gilmour) and the Max Planck Institute (MPI) of Colloids and Interfaces in Potsdam (led by Prof. Dr Peter H. Seeberger) has now developed a combined vaccine lead from synthetic fluorinated sugar molecules that is effective against both strains simultaneously. The vaccine lead is the component of the vaccine that triggers the desired immune response. The study has been published in the "Journal of the American Chemical Society".
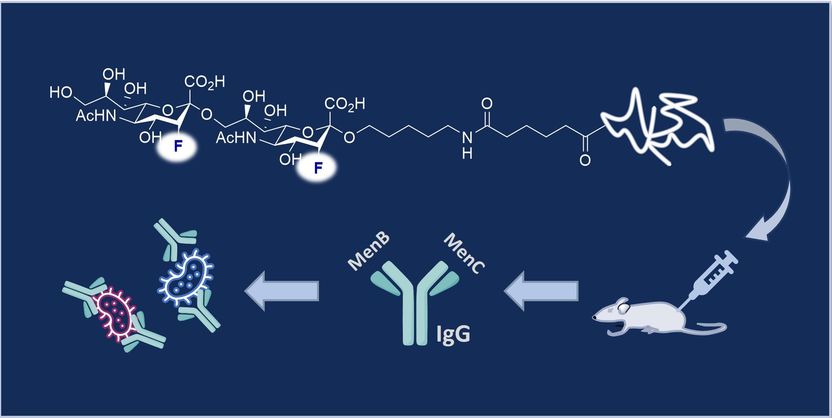
Chemists from the University of Münster and the Max Planck Institute (MPI) of Colloids and Interfaces in Potsdam have now developed a combined vaccine lead from synthetic fluorinated sugar molecules that is effective against meningococci B and C simultaneously.
© AK Gilmour
The introduction of fluorine into the natural structure brings many advantages, which begin with assisting in the synthesis and analysis, and enhancing metabolic stability. In this proof of concept study, the scientists demonstrated the efficacy of the lead compound in cell culture tests and in mice.
To produce the vaccine lead, the team mimicked the molecular fingerprint of the bacterial capsular polysaccharide (CPS) epitope. These polysaccharides form a shell around the bacteria, and their unique structures means that they function as antigens and can be specifically targeted by the immune response. This highly specific form of molecular recognition is the basis of a vaccine. The team synthesised a molecular structure that is closely similar to the epitope on the surface of the natural polysaccharide capsule and combined it with a carrier protein. Further investigations showed that this epitope analogue can trigger an immune response against serogroups B and C in mice.
The synthesis of the target molecule - a difluorinated analogue of the naturally occurring α-(2,9)-sialic acid epitope - required 16 steps and was carried out by Dr. Christina Jordan and PhD student Kathrin Siebold at the Institute of Organic Chemistry at the University of Münster. "There are many advantages to generating the key molecule through precision synthesis rather than isolating it from biological sources. The most obvious is that synthesis can be scaled up, and one can be sure that only the desired molecular structure is present in the vaccine lead", says Ryan Gilmour. "The exact composition is known and fully validated analytically, and the method is faster and cheaper than the isolation of native capsular polysaccharide. We are really excited that these fluorinated molecules are such effective mimics and that we have reached this translational point in our research. I think it underscores the societal impact of interdisciplinary research at the chemistry – biology interface."
PhD student Patricia Priegue (MPI) combined the fluorinated epitope inspired by serogroup C (MenC) with an outer cell membrane protein of serogroup B (MenB) to enable the production of a bivalent vaccine. The newly designed molecule triggered the production of highly specific immunoglobulin G (IgG) antibodies, subtype IgG1. "The antibodies generated by this strategy protected against meningococci B and C. We have validated this in vitro by SBA (serum bactericidal antibody) and OPKA assays (opsonophagocytic killing assay), which indicates that these fluorinated glycoconjugates are promising vaccine leads," emphasises Peter H. Seeberger.
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.





























































