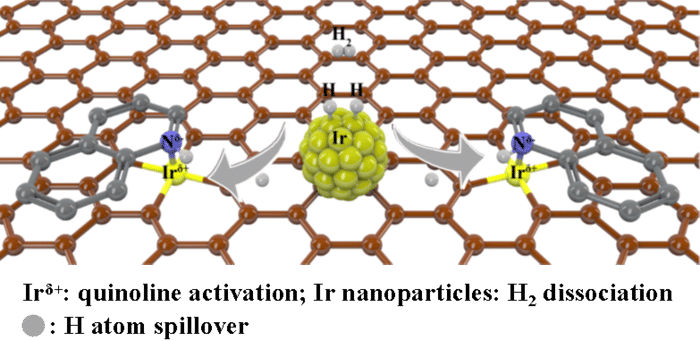
Safer alternative for an explosive reaction
The chemical industry has been using a reaction with explosive chemicals for over 100 years - now scientists have discovered a safer alternative
Explosions and poisoning. Serious injuries and even deaths. In the history of the chemical industry there have been repeated accidents, sometimes fatal, often caused by dangerous and explosive chemicals that are required for certain reactions.
Aryldiazonium salts, which have been used for 140 years, are such chemicals. They are very reactive and therefore extremely useful for producing other compounds – dyes, for example. However, the high reactivity means that isolated aryldiazonium salts are not very stable and can therefore react unintentionally and sometimes explosively. On December 23, 1969, there was a particularly serious explosion involving these chemicals at Ciba AG in Basel. A building was destroyed and heavy pieces of the reactor flew through the air. Three workers lost their lives and 31 were seriously injured. Despite such horrific reports, work continues with aryldiazonium salts.
A team led by Prof. Dr. Tobias Ritter, director at the Max Planck Institute for Kohlenforschung, has now succeeded in making the risky chemistry with aryldiazonium salts significantly safer. The Mülheim Protocol not only makes the use of these compounds less dangerous, but also potentially opens up opportunities for the development of new reactions.
“Normally, the use of diazonium salts takes place in two steps: you first isolate or accumulate the diazonium salt, which is dangerous, and then convert it into the desired product in a second step. In our project, we combine the two synthesis steps and go to the desired product without accumulating the diazonium salt, which significantly reduces the risk of an explosion,” explains Tim Schulte, a doctoral student from Tobias Ritter’s group.
Traditionally, aryldiazonium salts are synthesized from anilines with nitrous acid, or with nitrite compounds, a reaction that has experienced little innovation over the years. The reaction must be carried out at low temperatures (below 5 °C) because the aryldiazonium salts are unstable at higher temperatures. However, Javier Mateos, a postdoctoral researcher in the group, and Tim Schulte have discovered a new method that allows the presence of different nucleophiles in the reaction mixture.
The new strategy is based on the use of a natural process, nitrate reduction, which is carried out in plants. The researchers managed to imitate the natural process in a test tube and combine it with aryl diazonium chemistry to develop a safer synthesis method. This way, the above-mentioned limitations associated with traditional methods such as temperature sensitivity and the need for strong acids can be avoided.
Because the researchers combine several steps in their new protocol, large concentrations of the dangerous substance do not arise in the first place. And that's not all the scientists from Mülheim discovered: "For our synthesis method, we use chemicals that are used in large quantities in the fertilizer and fuel industries and are therefore inexpensive," says Tim Schulte. This could make the synthesis route extremely interesting for companies in the chemical industry as it means lower production costs.
“The solution to the problem could actually have been found 100 years ago, but the reaction as it has now been discovered would probably not have been planned the same way,” says Tobias Ritter. “The combination of chemicals that ultimately produces good results was discovered by chance while we were working on another project,” reveals Javier Mateos. Although the reagents used have been known for a long time, their potential for diazonium chemistry had simply been overlooked until now.
The new method is also exciting scientifically because new chemical approaches can now be pursued, that would not be readily possible with the classic method due to the high risk of explosion and instability of the connections.
Original publication
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
David_Rasnick
Saha_ionization_equation
Lee_Raymond
5-Hydroxyindoleacetic_acid
Clement_Clerke
Microparticles with feeling - Researchers develop a new method to simultaneously measure flow and oxygen - Accurate and fast as never before
Electromagnetic_radiation
Category:EC_1.14.15