Magnetic with a pinch of hydrogen
Research team develops new idea to improve the properties of ultra-thin materials
Magnetic two-dimensional materials consisting of one or a few atomic layers have only recently become known and promise interesting applications, for example for the electronics of the future. So far, however, it has not been possible to control the magnetic states of these materials well enough. A German-American research team led by the Helmholtz-Zentrum Dresden-Rossendorf (HZDR) and Dresden University of Technology (TUD) is now presenting in the journal Nano Letters an innovative idea that could overcome this shortcoming - by allowing the 2D layer to react with hydrogen.
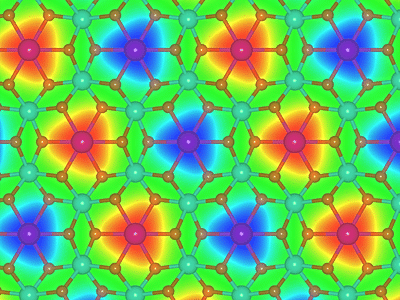
Magnetization density change of the non-van der Waals 2D material CdTiO3 upon hydrogenation with its passivated atomic structure superimposed onto it. Red regions indicate an enhancement of the magnetization whereas blue areas signal a related reduction.
HZDR / Tom Barnowsky
2D materials are ultra-thin, in some cases consisting of a single atomic layer. Due to their special properties, this still young class of materials offers exciting prospects for spintronics and data storage. In 2017, experts discovered a new variant – 2D materials that are magnetic. However, these systems have so far been difficult to switch back and forth between two magnetic states – a prerequisite for the construction of new types of electronic components – through targeted chemical influences. To overcome this problem, a research team from the HZDR and TUD led by junior research group leader Rico Friedrich set their sights on a special group of 2D materials: layers obtained from crystals in which relatively strong chemical bonds exist: so-called non-van der Waals 2D materials.
Twenty years ago, the later Physics Noble Prize winners Konstantin Novoselov and Andre Geim were able to produce a 2D material in a targeted manner for the first time: Using adhesive tape, they peeled off a thin layer from a graphite crystal, thereby isolating single-layer carbon, so-called graphene. The simple trick worked because the individual layers of graphite are only loosely bound chemically. Incidentally, this is exactly what makes it possible to draw lines on paper with a pencil.
"Only in recent years has it been possible to detach individual layers from crystals using liquid-based processes, in which the layers are much more strongly bound than in graphite," explains Rico Friedrich, head of the "DRESDEN-concept" junior research group AutoMaT. "The resulting 2D materials are much more chemically active than graphene, for example." The reason: these layers have unsaturated chemical bonds on their surface and therefore a strong tendency to bind with other substances.
Turn 35 into 4
Friedrich and his team came up with the following idea: if the reactive surface of these 2D materials were made to react with hydrogen, it should be possible to influence specifically the magnetic properties of the thin layers. However, it was unclear which of the 2D systems were particularly suitable for this. To answer this question, the experts combed through their previously developed database of 35 novel 2D materials and carried out detailed and extensive calculations using density functional theory. The challenge was to ensure the stability of the hydrogen-passivated systems in terms of energetic, dynamic and thermal aspects and to determine the correct magnetic state - a task that could only be accomplished with the support of several high-performance computing centers.
When the hard work was done, four promising 2D materials remained. The group took a closer look at these once again. "In the end, we were able to identify three candidates that could be magnetically activated by hydrogen passivation," reports Friedrich. A material called cadmium titanate (CdTiO3) proved to be particularly remarkable – it becomes ferromagnetic, i.e. a permanent magnet, through the influence of hydrogen. The three candidates treated with hydrogen should be easy to control magnetically and could therefore be suitable for new types of electronic components. As these layers are extremely thin, they could be easily integrated into flat device components – an important aspect for potential applications.
Experiments are already underway
"The next step is to confirm our theoretical findings experimentally," says Rico Friedrich. "And several research teams are already trying to do this, for example at the University of Kassel and the Leibniz Institute for Solid State and Materials Research in Dresden." But also at HZDR and TUD the research on 2D materials is continuing: among other things, Friedrich and his team are working on new types of 2D materials that could be relevant for energy conversion and storage in the long term. One focus is on the possible splitting of water into oxygen and hydrogen. The green hydrogen obtained this way could then be used, for example, as energy storage medium for times when there is too little solar and wind power available.