Hydrogen chloride can now be stored, processed, and electrolyzed safely thanks to breakthrough
New method paves the way for safer, more sustainable hydrogen and base chemical production
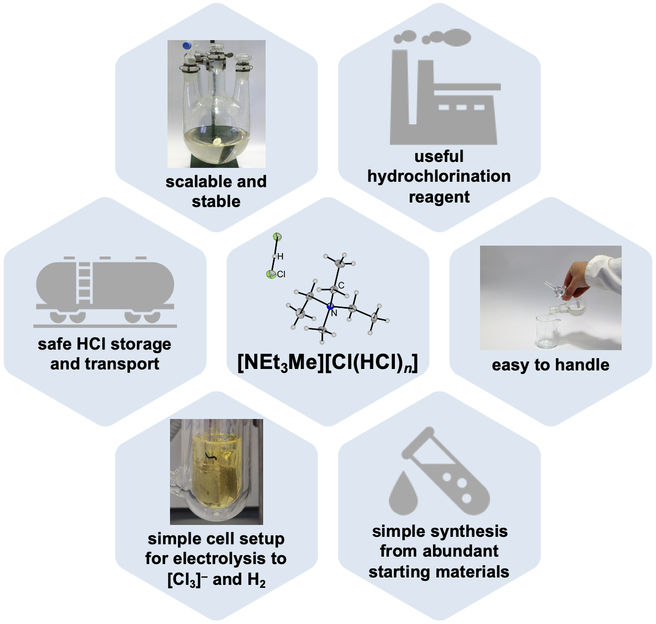
A research team at Freie Universität Berlin led by Professor Sebastian Hasenstab-Riedel has successfully developed a method for storing and electrolyzing gaseous hydrogen chloride in the form of an ionic liquid. The method allows the hydrogen chloride created as a by-product in traditional chlorination processes to be recovered and recycled in a safer manner. This development also represents an important contribution toward making the chemical industry more sustainable and offering alternative energy solutions. The results of the study were recently published in the scientific journal Science Advances under the title “Bichloride Based ionic liquids for the Merged Storage, Processing, and electrolysis of Hydrogen Chloride”.
Bichlorides enable simplified handling, storage and electrolysis of pure hydrogen chloride
Freie Universität Berlin/AG Hasenstab-Riedel
Hydrogen chloride (HCl) is an important by-product of the chemical industry – and can be electrolyzed to generate hydrogen and chlorine. Chlorine is one of the most important base chemicals, required for the production of indispensable polymers such as polyurethanes and polycarbonates, while hydrogen could be the key to the energy of the future. However, for this processing to occur, HCl must first be transported securely from industrial manufacturing plants to special electrolysis facilities powered by green energy. Due to the technically demanding nature of its secure transportation, such applications for HCl have largely been avoided to date. As such, the potential of this valuable resource has only been partially realized thus far.
Led by inorganic chemistry professor Sebastian Hasenstab-Riedel and in collaboration with partners from Technische Universität Berlin, a team of researchers at Freie Universität Berlin has developed a secure method of storing HCl. By converting it into an ionic liquid, hydrogen chloride can be handled more safely in its anhydrous (water-free) state. The researchers discovered that HCl gas can be safely bound to the salt triethylmethylammonium chloride to create an ionic liquid called “bichloride” under ambient conditions. It can then also be safely released from this bichloride following transportation or storage. This approach is not only safer than traditional techniques, its electrolysis also promises to be more energy efficient than traditional systems. Moreover, the bichloride can also be used to synthesize other base chemicals that can then be used to manufacture plastics or silicones.
“Chlorine and hydrogen chloride are gaining in importance when it comes to ushering in a more sustainable future for the energy-intensive chemical industry,” says Hasenstab-Riedel. He emphasizes that the technology developed by his team offers ideal conditions for storing and transporting hydrogen chloride in a cost-effective and secure manner. Hasenstab-Riedel adds, “This represents an important step toward producing chlorine in a more sustainable way, for example, with electricity produced through renewable energy.” At the same time, due to the industrial significance of chlorine, this system could also play an important role in stabilizing the power grid, thus supporting a transition to more sustainable energy practices. What is more, the ability to store hydrogen chloride as ionic liquids paves the way for a more sustainable way of performing fundamental chemical processes.
Original publication
Most read news
Original publication
Gesa H. Dreyhsig, Patrick Voßnacker, Merlin Kleoff, Haralds Baunis, Niklas Limberg, Michael Lu, Reinhard Schomäcker, Sebastian Riedel; "Bichloride-based ionic liquids for the merged storage, processing, and electrolysis of hydrogen chloride"; Science Advances, Volume 10
Organizations
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.





























































