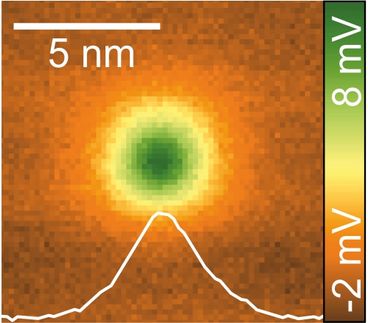
Looking at the Sides of Molecules: Lateral force microscopy reveals previously unseen hydrogen atoms
The ability to directly observe hydrogen atoms marks a significant breakthrough and opening up new avenues for research and innovation
Researchers at the University of Regensburg and the Graz University of Technology have shown that hydrogen atoms at the sides of molecules lying on a surface can directly be seen. The study, published in the journal “Proceedings of the National Academy of Sciences”, describes that by looking beside the molecules, the position and presence of the previously-hidden hydrogen atoms could be revealed.
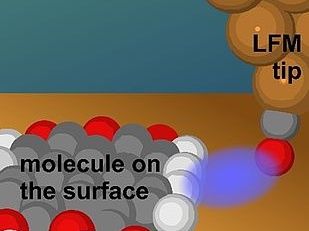
Artists rendition of a LFM tip coming close to the side of a molecule, where it is sensitive to the terminal H-atoms.
© A. J. Weymouth
Hydrogen atoms situated at the edges of molecules affect many properties of those molecules, including how they interact with other molecules. Hydrogen bonds are one of the most common forms of molecular interactions, in which a positively-charged hydrogen atom at the side of a molecule is attracted to a negative atom in a neighbouring molecule. Hydrogen bonds are of great importance in the field of on-surface synthesis, in which molecules are first absorbed onto a surface and then react with each other. But despite their significance, direct observations of these small yet important atoms have been elusive.
To visualize the sides of molecules, researchers employed a specialized technique derived from Atomic Force Microscopy (AFM). In AFM, a sharp tip is brought close to a surface, and the forces on the tip are recorded as it moves over the surface. Previous AFM experiments focussed on the vertical component of force and had not revealed the hydrogen atoms at the sides of molecules. To overcome this limitation, researchers employed Lateral Force Microscopy (LFM), which measures the horizontal forces exerted on the AFM tip. PD Dr Alfred J. Weymouth from the working group of Prof. Dr Franz J. Gießibl, holder of the Chair of Quantum Nanoscience at the UR, is a leading expert in the field of LFM. He highlighted its unique capabilities, stating, "despite the fact that it is not widely used, LFM offers several advantages over conventional AFM, including exceptional distance sensitivity, enabling the extraction of physical parameters from a single image, and the ability to quantify frictional forces by sliding a single atom across chemical bonds."
By measuring the lateral force exerted on the AFM tip at the edges of the molecules, Dr Weymouth and coworkers were able to directly visualize the hydrogen atoms. The raw data from the experiments could be compared directly to theoretical calculations, providing a deeper understanding of the atomic interactions at play. While atom-atom interactions are often modeled using simplified distance-dependent functions, comparing these models to the experimental data revealed the limitations of these approximations, highlighting the importance of incorporating additional factors into these theoretical frameworks. This insight is valuable for both AFM and LFM investigations, as it allows researchers to refine their understanding of basic atomic interactions.
The ability to directly observe hydrogen atoms marks a significant breakthrough for researchers, providing a powerful tool to elucidate the intricate mechanisms and intermediate steps of on-surface chemical reactions. This advancement holds immense potential for accelerating progress in various fields, including surface catalysis and molecular interactions within the human body. The development of this novel technique represents a significant step forward in our understanding of the microscopic world, opening up new avenues for research and innovation. By directly visualizing the behavior of hydrogen atoms, researchers can gain deeper insights into the fundamental processes that govern the interactions of molecules, paving the way for transformative advancements in various fields.
Original publication
Original publication
Shinjae Nam, Elisabeth Riegel, Lukas Hörmann, Oliver T. Hofmann, Oliver Gretz, Alfred J. Weymouth, Franz J. Giessibl; "Exploring in-plane interactions beside an adsorbed molecule with lateral force microscopy"; Proceedings of the National Academy of Sciences, Volume 121, 2024-1-3
Topics
Organizations
Other news from the department science

Get the chemical industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents

acker raum-systeme gmbh - Hamburg, Germany
Johann_Friedrich_Schweitzer

COMP MALL Computer-Vertriebs GmbH - München, Germany

Nature's toughest substances decoded
Category:Georgian_chemists
Category:Tandem_mass_spectrometry
'Dancing' holes in droplets submerged in water-ethanol mixtures