Process for the optical analysis of trace gases optimised
Prof. Gernot Friedrichs from Kiel University has developed a new approach for making interfering signals in laser absorption spectroscopy invisible
Laser-based absorption spectroscopy is an important method for determining the concentration of gas components in a sample. Modern devices are highly specialised for detecting very specific gases, such as trace gases in the atmosphere, in combustion exhaust fumes and in technical applications of plasmas. In order to do so, they measure the proportion of light of a specific wavelength that is absorbed or attenuated by a sample. This enables the concentration of the gas to be determined. The selected detection wavelength depends on which molecule is to be measured. A common problem is that different molecules can absorb the same light – even at a cleverly chosen wavelength. “The absorption spectra of the different gas molecules sometimes overlap very significantly. This means that if I want to detect molecule A, I also always get a varying degree of strong signal from molecule B,” explained Professor Gernot Friedrichs from the Institute of Physical Chemistry at Kiel University (CAU). This so-called cross-sensitivity limits the effectiveness of the measurement method. To date, this problem has either been eliminated or at least reduced by additional measurements at different wavelengths, i.e. the measurement of spectra, or the interfering gases are separated by means of gas chromatography methods before the actual measurement. Friedrichs and his former doctoral candidate, Dr Ibrahim Sadiek from the Leibniz Institute for Plasma Science and Technology e.V. (INP), Greifswald, have now demonstrated that there is an easier solution. They have developed a method to overcome this cross-sensitivity in absorption spectroscopy, even when measurements are only conducted at one wavelength. The feasibility study for the new, patent-pending two species-one wavelength (2S1W) method based on selective optical saturation was recently published in the scientific journal Scientific Reports.
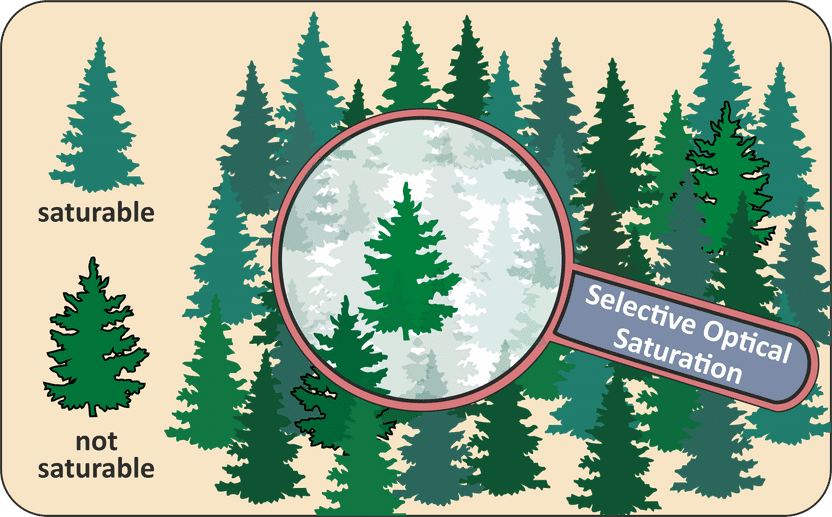
The detection of very low concentrations of trace gases in the atmosphere can be compared with the search for a few fir trees in a spruce forest. The image illustrates that selective optical saturation can be used to make the undesired information – in this case, the spruce trees – disappear as if concealed
© Illustration: Gernot Friedrichs, Kiel University
Eliminating interfering signals using optical saturation
The new method exploits the phenomenon of optical saturation in molecules. The state of optical saturation only occurs at high light intensities, which can nowadays be generated quite easily with lasers. The molecules then become “transparent” for absorption spectroscopy, meaning that the irradiated light is no longer attenuated. The point at which the sample becomes transparent is a property of the respective gas type. Until now, optical saturation was considered disadvantageous for absorption measurements and thus avoided as much as possible, since it distorts the concentration measurement. However, Sadiek and Friedrichs have now demonstrated in their study that taking advantage of selective optical saturation can even help to separately determine the concentrations of two completely mutually-interfering molecules at a fixed wavelength. “To do so, we varied the light intensity very rapidly and over a wide range in a special measuring cell. At low light intensity, the sum of the absorptions of both species is measured, and at high light intensity, one of the molecules is saturated. So we only detected the signal from one species. In our case, the methyl chloride was detected, since the methane was already saturated,” emphasised Sadiek. “When we first tried this, we were fascinated by how well it actually works to separate the signals from both species in this conceptually simple way.”
A typical problem in practice is, for example, detecting chlorinated hydrocarbons, which occur in very low concentrations in the atmosphere. “If you want to detect them without separating the mixture beforehand, you automatically encounter the problem that the trace gases present in higher concentrations, such as carbon dioxide or methane and especially water vapour, i.e. the humidity, interfere with the measurement. With our method, we can simply make these interfering gases invisible in the spectrum,” explained Friedrichs. His group is currently working on marine research projects to further develop the method for use in conventional absorption spectrometers.The potential for reducing cross-sensitivities will then be demonstrated in field measurements in order to better investigate exchange processes at the water-air interface. In principle, the method is also suitable for the simultaneous detection of a large number of trace gases, provided that they have a sufficiently different saturation intensity.
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!

Topic World Spectroscopy
Investigation with spectroscopy gives us unique insights into the composition and structure of materials. From UV-Vis spectroscopy to infrared and Raman spectroscopy to fluorescence and atomic absorption spectroscopy, spectroscopy offers us a wide range of analytical techniques to precisely characterize substances. Immerse yourself in the fascinating world of spectroscopy!