Physicists create giant trilobite molecules
Results are important to understand the chemical binding mechanisms of them, which is distinct from all other chemical bonds
Advertisement
Kaiserslautern physicists in the team of Professor Dr. Herwig Ott have succeeded for the first time in directly observing pure trilobite Rydberg molecules. Particularly interesting is that these molecules have a very peculiar shape, which is reminiscent of trilobite fossils. At the same time, they have the largest electric dipole moments of any molecule known so far. The researchers used a dedicated apparatus, which is capable to prepare these fragile molecules at ultralow temperatures. The results are important to understand the chemical binding mechanisms of them, which is distinct from all other chemical bonds. The study was published in the renowned journal “Nature Communications”.
For their experiment, the physicists used a cloud of rubidium atoms that was cooled down in an ultra-high vacuum to about 100 microkelvin – 0.0001 degrees above absolute zero. Subsequently, they excited some of these atoms into a so-called Rydberg state using lasers. “In this process, the outermost electron in each case is brought into far-away orbits around the atomic body”, explains Professor Herwig Ott, who researches ultracold quantum gases and quantum atom optics at University of Kaiserslautern-Landau. “The orbital radius of the electron can be more than one micrometer, making the electron cloud larger than a small bacterium.” Such highly excited atoms are also formed in interstellar space and are chemically extremely reactive.
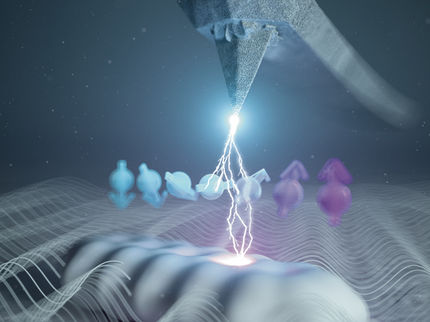
If a ground state atom is now located within this giant Rydberg atom, a molecule is formed. While standard chemical bonds are either covalent, ionic, metallic or of dipolar nature, the trilobite molecules are bound by a completely different mechanism. “It is the quantum mechanical scattering of the Rydberg electron from the ground state atom, which sticks the two together” says Max Althön, who is first author of the study. Althön explains further: “Imagine the electron rapidly orbiting around the nucleus. On each round trip, it collides with the ground state atom. In contrast to our intuition, quantum mechanics teaches us that these collisions lead to an effective attraction between the electron and the ground state atom.”
The properties of these molecules are amazing: Due to the wave nature of the electron, the multiple collisions lead to an interference pattern which looks like a trilobite. Moreover, the bond length of the molecule is as large as the Rydberg orbit – way bigger than any other diatomic molecule. And because the electron is so strongly attracted by the ground state atom, the permanent electric dipole moment is extremely large: more than 1700 Debye.
In order to observe these molecules, the scientists have developed a dedicated vacuum apparatus. It allows for preparing ultracold atoms via laser cooling and subsequent spectroscopic detection of the molecules. The results help to understand fundamental binding mechanisms between ground state atoms and Rydberg atoms, which have recently become also a promising platform for quantum computing applications. The discovery of the researchers complements the understanding of Rydberg systems, which can be exotic and useful at the same time.