Competitor for diamonds: Scientists produce superhard multifunctional carbon nitrides
Advertisement
In a groundbreaking piece of research, scientists have synthesised long-sought carbon nitrogen compounds and unlocked the potential of carbon nitrides as a new class of superhard multifunctional materials that could rival diamond. The work has now been published in the journal Advanced Materials.
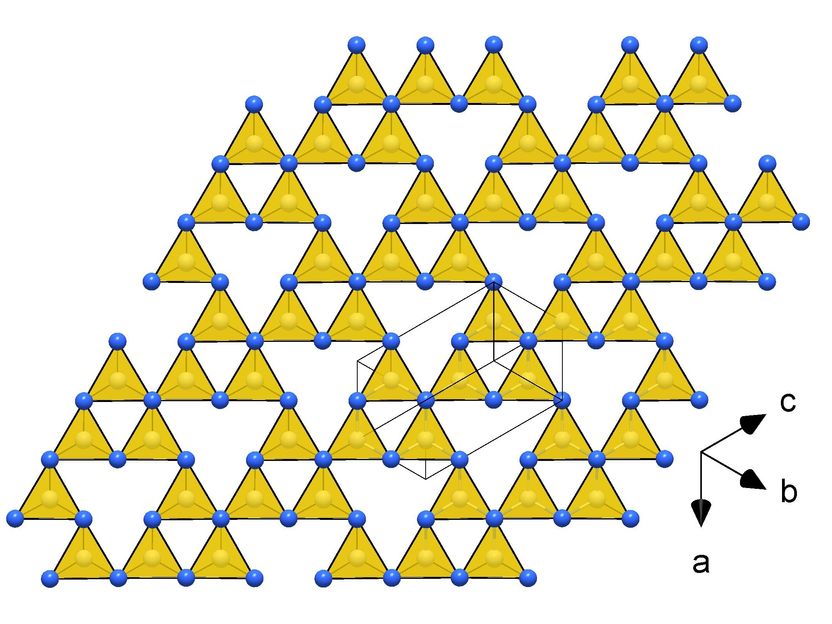
Frameworks of CN₄ tetrahedra in the crystal structures of novel carbon nitrides making them ultraincompressible and superhard: tI14-C₃N₄
Universität Bayreuth
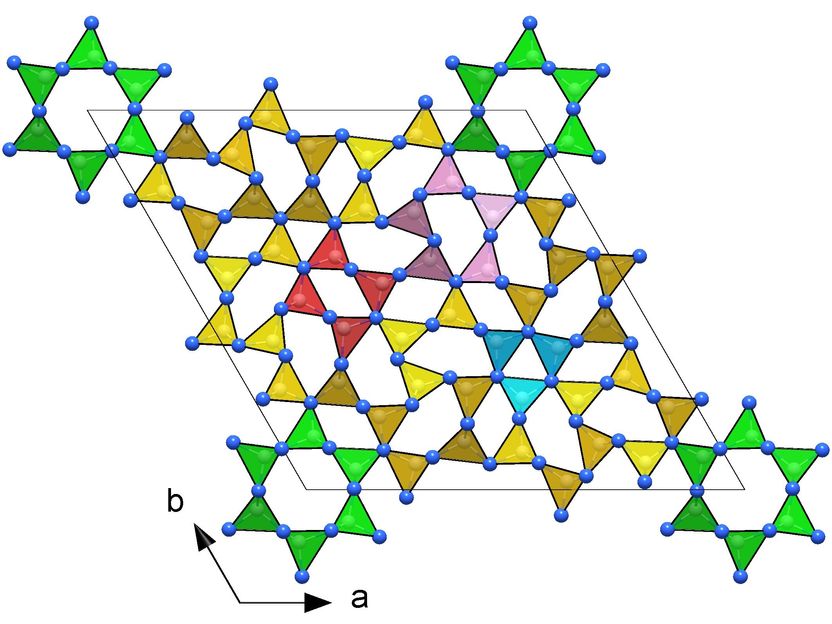
Frameworks of CN₄ tetrahedra in the crystal structures of novel carbon nitrides making them ultraincompressible and superhard: hP126-C₃N₄ (right).
Universität Bayreuth
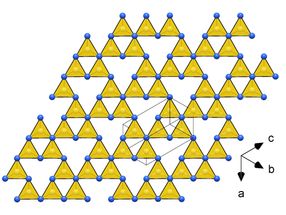
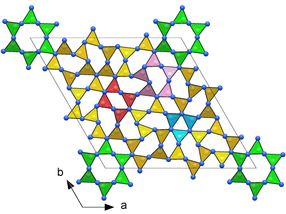
Since 1989, when a prediction of a carbon-nitrogen compound C₃N₄ with exceptional mechanical properties, potentially surpassing diamond in hardness was reported in the journal Science, researchers worldwide have been working on this topic. The breakthrough has now been achieved by an international team of high-pressure scientists from the University of Bayreuth and the University of Edinburgh.
They subjected various carbon-nitrogen precursors to incredibly high pressures between 70 and 135 gigapascals (GPa), with 100 GPa corresponding to 1,000,000 times the atmospheric pressure, and heated them above 2000 K in diamond anvil cells. The samples were then characterized by single-crystal X-ray diffraction at three particle accelerators: the European Synchrotron Research Facility (ESRF, France), the Deutsches Elektronen-Synchrotron (DESY, Germany) and the Advanced Photon Source (APS, United States). The results revealed four carbon nitrides with the compositions CN, CN₂, and C₃N₄, and structures of different complexity. The crystal structures of the C₃N₄ allotropes are built of frameworks of corner-sharing CN₄ tetrahedra, that is a key to their superior mechanical properties - ultraincompressibility (incompressibility manifests when the volume of a body remains almost constant despite applied pressure) and superhardness - experimentally established in this work. The fact that the high-pressure C₃N₄ carbon nitrides make imprints on a diamond surface give evidence of their hardness comparable to diamond itself.
“The carbon nitrides synthesized in this work are expected to exhibit multiple exceptional functionalities besides their mechanical properties, with a potential to be engineering materials in the same category as diamond, but unlike diamond, they can be easily doped, what is always an issue with diamond electronics,” says Professor Natalia Dubrovinskaia of the Laboratory of Crystallography at the University of Bayreuth, a senior author of the research. Physical properties investigations, both experimental and theoretical, the latter conducted by the scientists of the University of Linköping, Sweden, showed that these strongly covalently bonded materials are not only ultra-incompressible and superhard, but also possess high energy density, piezoelectric, photoluminescent, and nonlinear optical properties.
Remarkable is also that all four high-pressure carbon nitrides can be recovered to ambient pressure and temperature. “The recovery of complex materials synthesized above 100 GPa is a previously unprecedented case, thus opening up new perspectives for high-pressure materials science in general”, says Professor Leonid Dubrovinsky of the Bavarian Institute for Experimental Geochemistry and Geophysics at the University of Bayreuth, the leading author of the research.
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.

Topic world Synthesis
Chemical synthesis is at the heart of modern chemistry and enables the targeted production of molecules with specific properties. By combining starting materials in defined reaction conditions, chemists can create a wide range of compounds, from simple molecules to complex active ingredients.